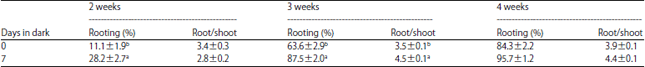
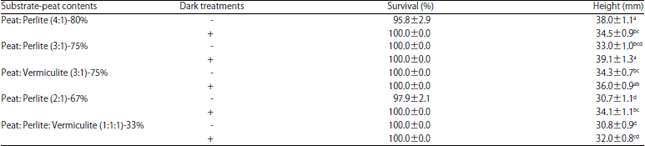
Research Article
Large-Scale Micropropagation of Common Ash
Group of Forest Biotechnology, Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry Russian Academy of Sciences, Pushchino, Moscow Region, Russia
Konstantin Shestibratov
Group of Forest Biotechnology, Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry Russian Academy of Sciences, Pushchino, Moscow Region, Russia