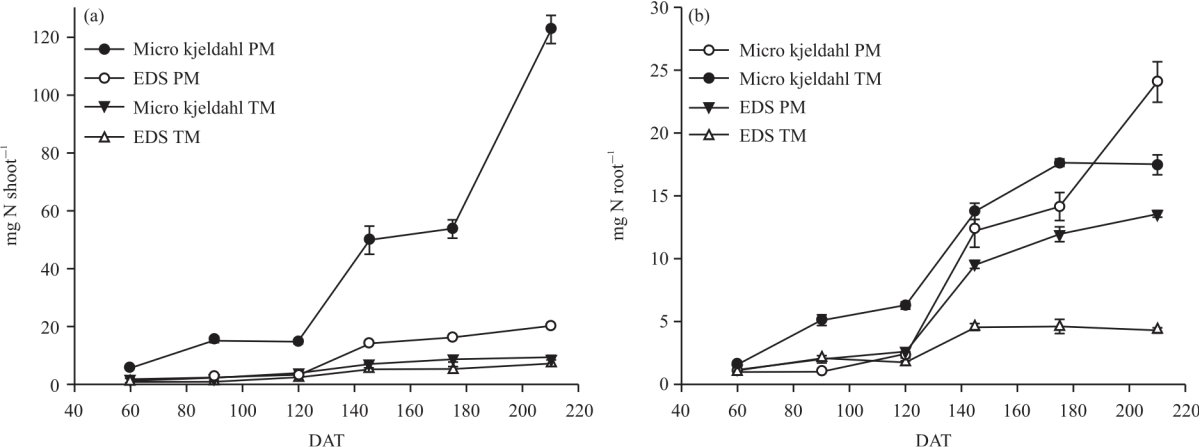
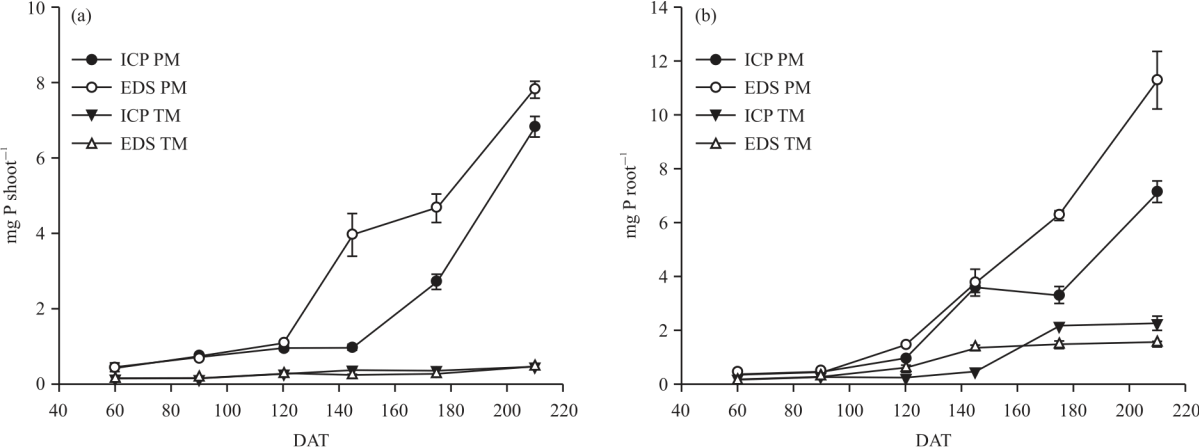
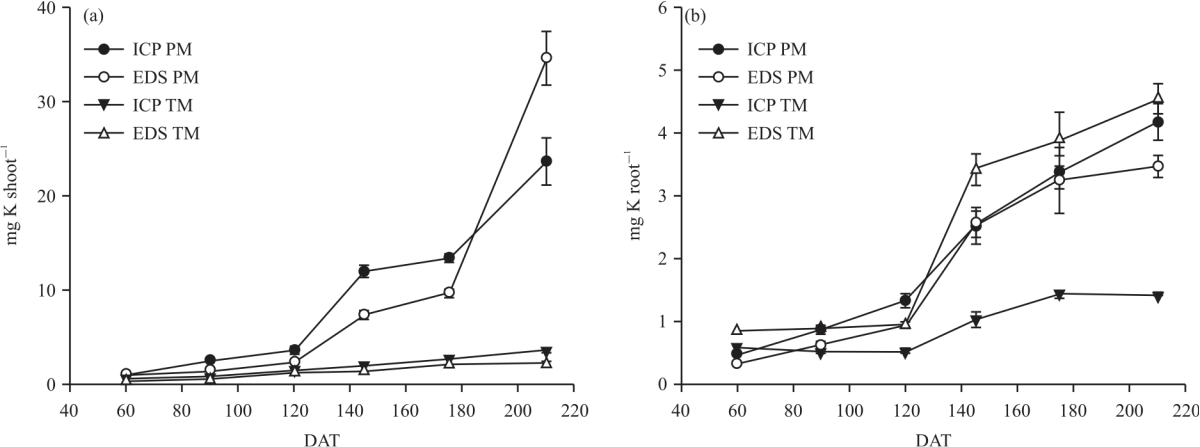
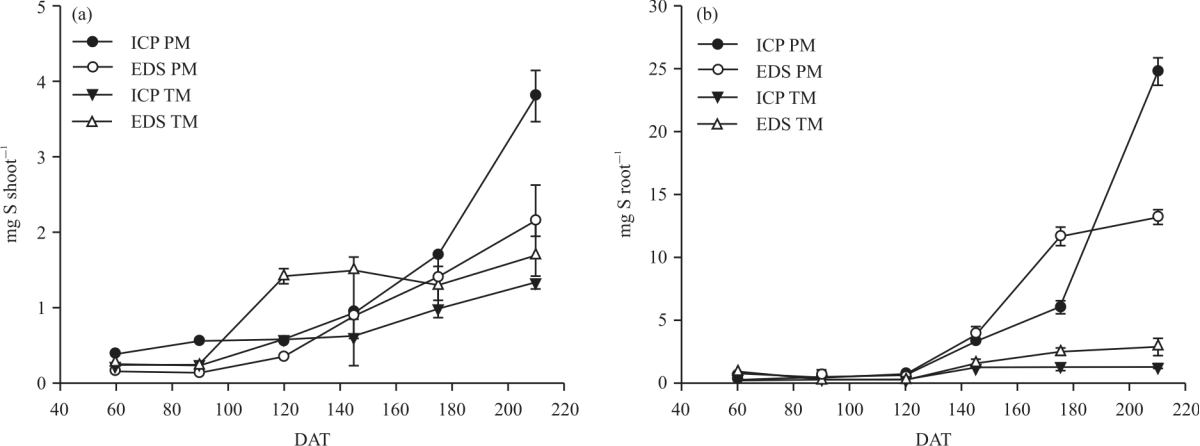
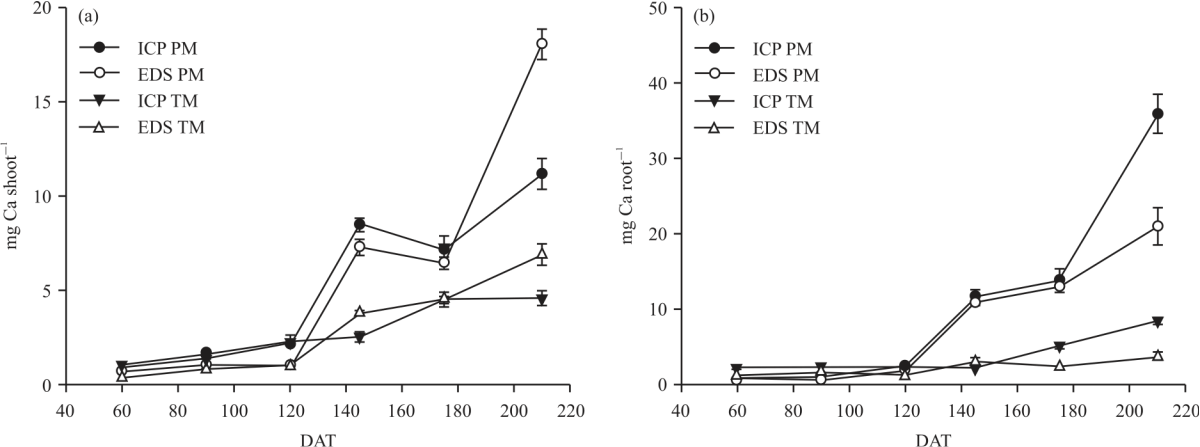
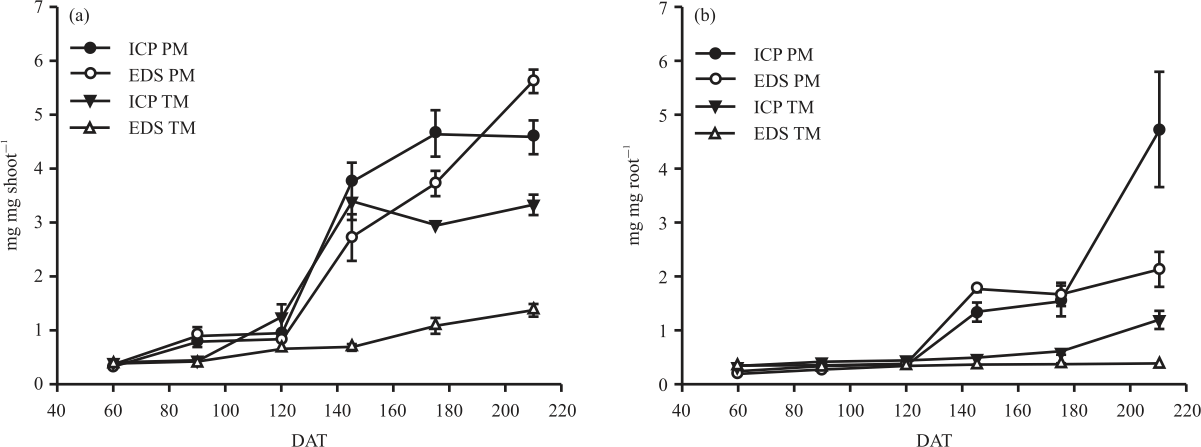
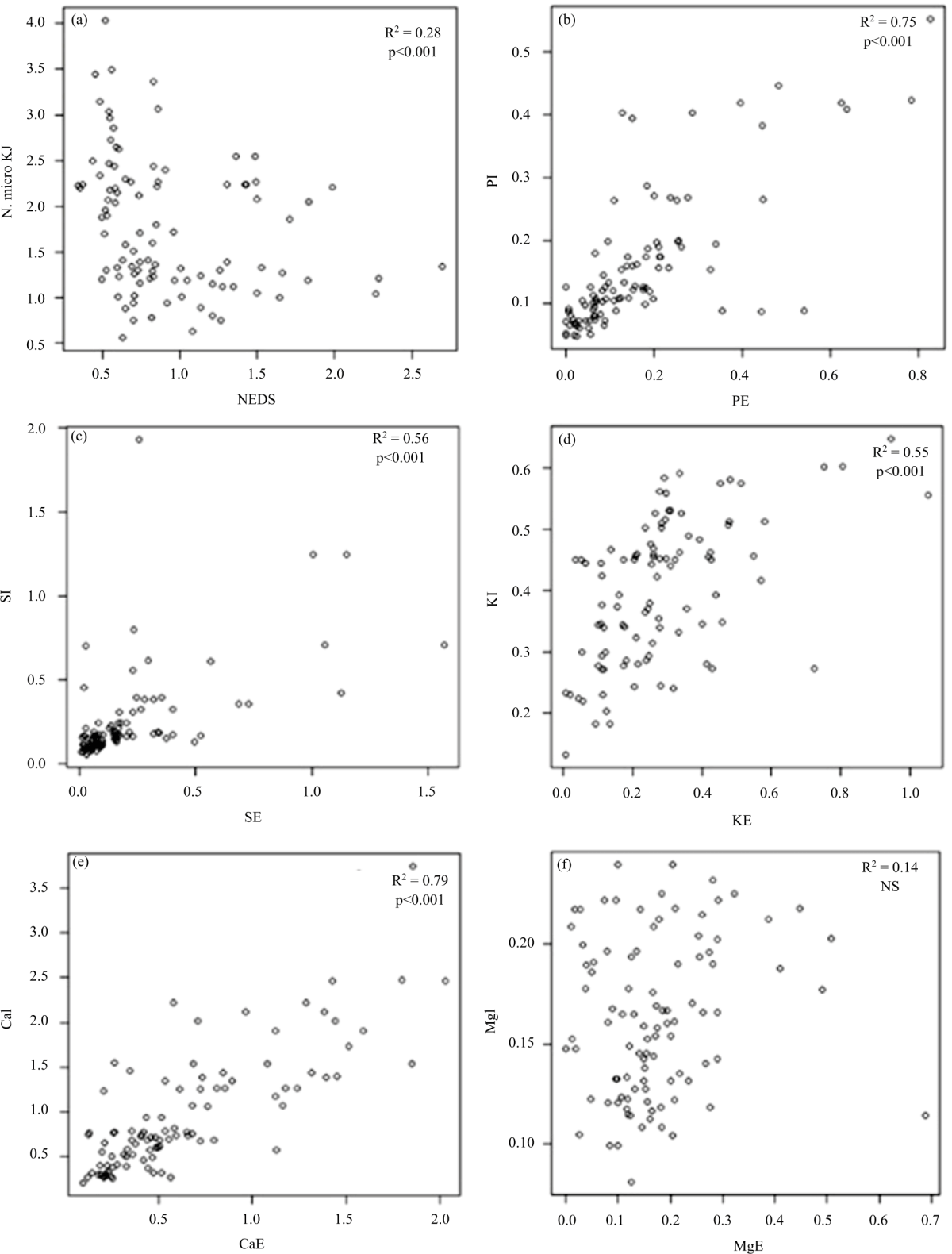
Research Article
Determination of Nutrients by SEM-EDS and ICP-OES in Mexican Pine Pinus greggii var. greggii
Department of Horticulture, Universidad Autónoma Agraria Antonio Narro, México
Fernando Galindo-García
Department of Horticulture, Universidad Autónoma Agraria Antonio Narro, México
Willian Narvaez-Ortiz
Department of Horticulture, Universidad Autónoma Agraria Antonio Narro, México
Rosalinda Mendoza-Villarreal
Department of Horticulture, Universidad Autónoma Agraria Antonio Narro, México
Eduardo San Martin-Martinez
Instituto Politecnico Nacional, Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada Unidad Legaria, Ciudad de México, México
LiveDNA: 52.34395