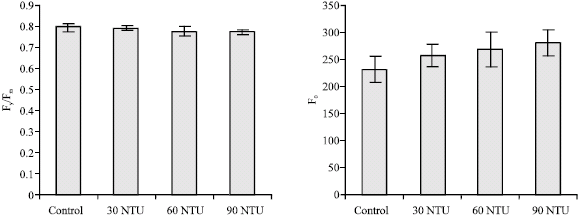
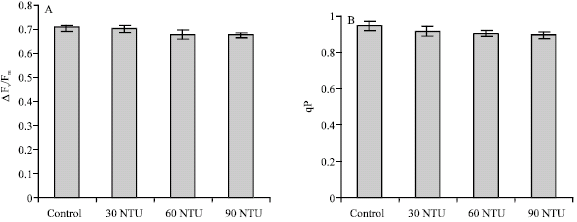
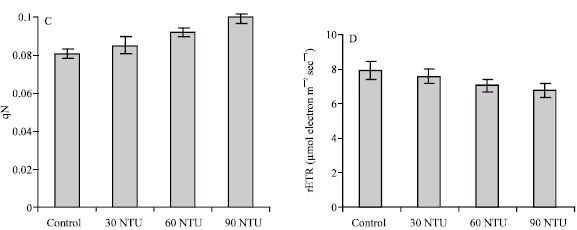
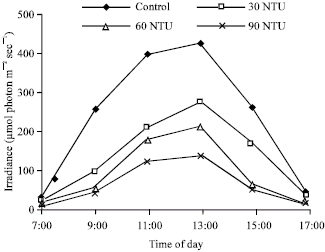
Research Article
Effects of Water Turbidity on the Photosynthetic Characteristics of Myriophyllum spicatum L.
Jiangsu Key Laboratory of Environmental Change and Ecological Construction, College of Geographical Science, Nanjing Normal University, Nanjing 210097, China
Wang Guoxiang
Jiangsu Key Laboratory of Environmental Change and Ecological Construction, College of Geographical Science, Nanjing Normal University, Nanjing 210097, China
Li Qiang
Jiangsu Key Laboratory of Environmental Change and Ecological Construction, College of Geographical Science, Nanjing Normal University, Nanjing 210097, China