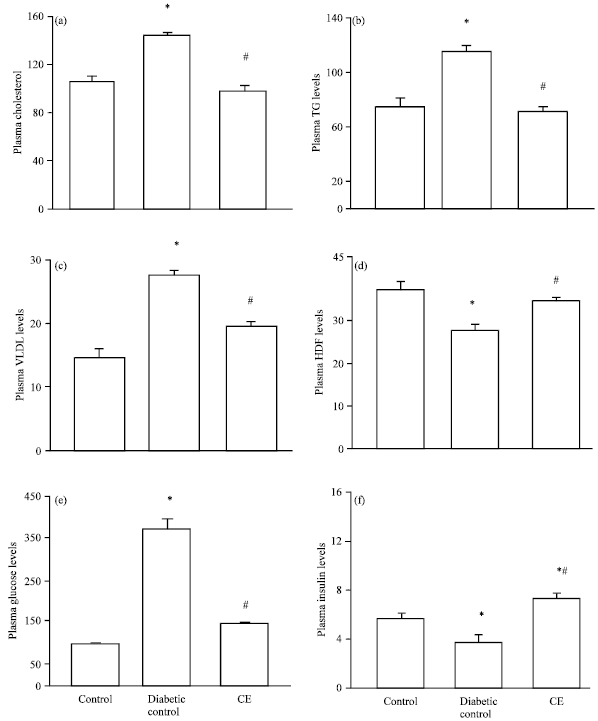
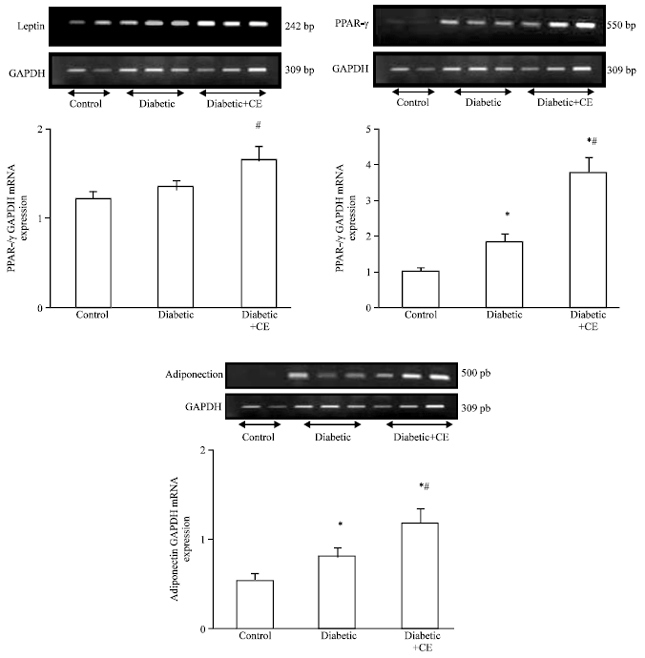
Research Article
Biomedical Effects of Cinnamon Extract on Obesity and Diabetes Relevance in Wistar Rats
Medical Laboratories, Turabah and College of Science, Taif University, Saudi Arabia
Hossam Fouad Attia
Medical Laboratories, Turabah and College of Science, Taif University, Saudi Arabia
Samir Ahmed El-Shazly
Department of Biochemistry and Histology, Benha University, Moshtohor, P.O. 13736 and Kafr El-Sheikh University, Egypt
Osama Mesilhy Saleh
Department of Biochemistry and Histology, Benha University, Moshtohor, P.O. 13736 and Kafr El-Sheikh University, Egypt