Research Article
Formation of Highly Antioxidative Liposomes from Crude Acetone Extracts of Canna indica, Cucumis melo and Prunus armeniaca
Department of Biological Sciences, Mutah University, P.O. Box 7, Mutah, Jordan
LiveDNA: 962.11902
Liposomes have been studied and characterized for over 50 years since first being described in the 1960s (Bangham et al., 1965). These vesicles are composed of a lipid bilayer that spontaneously forms in the presence of water (Mozafari, 2005). The amphiphilic characteristics of liposomes and ease of vesicle formation (Grabielle-Madelmont et al., 2003) allows them to encapsulate molecules and serve as carriers for various biologically active compounds (Felnerova et al., 2004; Brgles et al., 2009), such as vitamins, enzymes, drugs, proteins, nucleic acids (Brgles et al., 2007) and some vaccines (Chen et al., 2008).
Liposomes have been used as a drug carrier to improve the therapeutic activity and safety of drugs by altering their pharmacokinetics (Lian and Ho, 2001), solubility, bioavailability and stability as well as reducing their toxicity and enhancing targeting to their site of action. Furthermore, due to their biodegradability and low permeability to small hydrophilic molecules, liposomes have been used in many areas such as cancer chemotherapy, ophthalmology, antimicrobial therapy, vaccines, gene therapy and diagnostic applications (Allen et al., 1995).
The lipid composition of liposomes plays a significant role in determining their phase behavior as well as other properties such as permeability, fusion, aggregation and stability, which can all affect their efficacy in delivery and biological systems (Barenholz, 2001), interaction with cells in vivo and in vitro and the encapsulation efficiency of various substances, especially drugs (Volodkin et al., 2007). Many studies have attempted to enhance liposomal stability and minimize oxidative degradation, because stability is a prerequisite for the exploitation of liposomes in the delivery of therapeutic molecules (Sivakumar and Rao, 2003; Lau et al., 2005). Several methods were done to enhance stability include preparation of more stable bilayers, coating the liposome surface with protective polymers, addition of polyethylene glycol, preparation of polymerized liposomes and charge modification and freeze drying (EI-Samaligy et al., 2006).
Other strategies involve the addition of various molecules such as Alkylresorcinols (ARs), vitamins, chitosan and bovine serum albumin to minimize phospholipid oxidation and modify liposomal behavior (Guo et al., 2003; Takeuchi et al., 2001). ARs are polyketide-derived compounds (Alastair et al., 2004) and are defined as amphiphilic 1, 3-dihydroxybenzene derivatives with an odd-numbered alkyl chain at position 5 of the benzene ring. ARs have been reported to have anticancer, antimicrobial, antiparasitic, antitumour, antioxidant, antifungal, antileukemic, enzyme-inhibiting and DNA-cleaving properties (Francisco et al., 2005). In addition, ARs are amphiphilic, exhibit strong affinity for lipid bilayers and biological membranes and can affect membrane structure and properties (Stasiuk and Kozubek, 2008). ARs are abundant in plant seeds, fruits, higher plants, algae, mosses, fungi and bacteria (Zarnowski et al., 2001). The antioxidant properties of ARs may play a role in protecting both free fatty acids and phospholipids against peroxidation, auto-oxidation and oxidation of biological membranes (Deszcz and Kozubek, 2000). Canna indica, Cucumis melo and Prunus armeniaca are known to have antioxidant activity and the extract of these plants may be used as a main component of liposomes to enhance liposome stability, therapeutic activity and to be used as a delivery or carrier system for the treatment of many diseases.
In this study, the phospholipids and ARs content in acetone extracts of seeds of Canna indica, Cucumis melo and Prunus armeniaca was determined. These crude acetone extracts are composed of mixtures of individual phospholipids, ARs and other compounds and were used to form highly antioxidative liposomes. In addition, the interaction of the liposomes with white blood cells was characterized.
Materials: Plant fruit of Canna indica, Cucumis melo and Prunus armeniaca were collected in may 2009 from local markets. The research project was conducted from May 2009 to August 2011.
Acetone extraction of Canna indica, Cucumis melo and Prunus armeniaca: Fifty grams of each type of plant seed were ground in a coffee grinder. The resulting powder was then soaked with acetone and extracted by continuous shaking for 25 h at Room Temperature (RT). The extracts were filtered and the residues soaked again with acetone for an additional 25 h at RT. The extracts were then filtered and combined and the final extracts were evaporated to dryness in a rotary evaporator (RV 05-ST Janke and Kunkel, IKA, Germany) to form a dry film.
Qualitative and quantitative analysis: The dry films of the extracted material were dissolved in chloroform (1 mL). The extracts were then qualitatively analyzed by Thin-layer Chromatography (TLC). Two developmental systems for TLC were used: chloroform: ethyl acetate (85:15, v/v) to separate ARs, which were specifically detected by immersing the plate for 5-10 sec in Fast Blue B solution (Sigma-Aldrich Chemie GmbH, Germany) to yield pink-red spots (Kulawinek et al., 2008) and chloroform:methanol:distilled water (65:25:4, v/v/v) to separate phospholipids, which were specifically detected by immersing the plate for 1-2 min in ammonium molybdate solution to yield blue spots (Atrouse and Qato, 2000).
Phospholipids content was measured using a phosphate assay according to the method described by Rouser et al. (1970). ARs content was determined according to the method described by Sampietro et al. (2009).
Liposome preparation and optical densities: Phosphatidylcholine (Phospholipid GmbH, Cologne, Germany) (5 mg mL-1) in chloroform solution was evaporated using the rotary evaporator. The lipid film was hydrated with Phosphate-buffered Saline buffer (PBS) (100 mM, pH 7.4). Liposomes were formed by immersion in an ultrasonication bath (Clifton, England) for 90 min (Kagan et al., 1990). The dry films prepared from the crude acetone extracts of Canna indica, Cucumis melo and Prunus armeniaca were hydrated with PBS and sonicated as described above. The optical densities of the liposome suspensions were measured at wavelengths ranging from 350-750 nm (Trofimove and Nisnevich, 1990) using a Vis-Spectrophotometer (Biotech Engineering Management Co. LTD, UK).
Liposome peroxidation assay (thiobarbituric acid method): To 500 μL liposome solution, 500 μL PBS (100 mM, pH 7.4) and 100 μL FeSO4.7H2O (4 mM) were added, followed by addition of 100 μL ascorbic acid (2 mM). After incubation for 30 min at 37°C, the reaction was terminated by the addition of 5.5% trichloroacetic acid. Next, 250 μL of Thiobarbituric Acid (TBA) solution (in 50 mM NaOH) was added to 1 mL of the above reaction mixture, followed by heating for 10 min. The mixtures were centrifuged at 3000 rpm for 10 min and the supernatant absorbance was determined at 532 nm (Choi et al., 2002). The inhibition ratio (%) was calculated using the following formula:
where, A was the absorption of the control and As was the absorption of the sample.
Cell isolation: Blood samples (with EDTA) obtained from volunteers were centrifuged and the buffy coat layer was removed and washed three times with PBS. Then, 1 mL 0.155 M ammonium chloride was added to lyse the residual erythrocytes. The White Blood Cell (WBC) viability was determined to be 95-98% by stained blood films (Wright stain). The WBCs were then suspended in PBS (Nilsson and Palmblad, 1988).
Superoxide ion production: Free radical production was determined by measuring the reduction of Nitro Blue Tetrazolium (NBT) to Formazan (Tonetti et al., 1991). In brief, cells (1x106 mL-1) were incubated with liposomes for 10 min at 37°C, followed by the addition of 50 μL NBT solutions. After incubation for 20 min at 37°C, the reaction mixture was centrifuged at 1000 rpm for 10 min. The absorbance was determined at 520 nm.
Statistical analysis: All analyses were performed on triplicate samples. The results were expressed as Mean±SD. The Student’s t-test was used for the evaluation of statistical significance (p<0.05).
Qualitative TLC analysis of Canna indica, Cucumis melo and Prunus armeniaca acetone extracts showed that they contained PLs, ARs and other compounds.
| Table 1: | Determination of PLs and ARs content obtained from acetone extracts of Canna indica, Cucumis melo and Prunus armeniaca using the phosphate assay method and micro method, respectively |
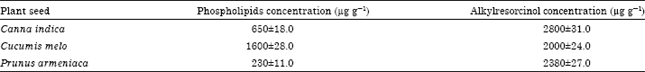 | |
| Data represented as Mean±SD, Values are statistically significant at p<0.05 | |
The extracted materials were then separated by TLC and developed with different methods to yield spots that were specifically stained with ammonium molybdate or Fast Blue B to detect the presence of PLs and ARs, respectively. These qualitative experiments were followed by quantitative determinations of the PLs content using a phosphate assay and the ARs content using the micro method (Table 1). Acetone extract from Canna indica, Cucumis melo and Prunus armeniaca contained 650, 1600 and 230 μg g-1 PLs and 2800, 2000 and 2380 μg g-1 ARs, respectively. These results illustrate that there were large differences in the quantities of PLs in each extract and that Cucumis melo contained the largest amount of PLs followed by Canna indica and Prunus armeniaca. Smaller differences were observed in the quantities of ARs with Canna indica having the largest amount followed by Prunus armeniaca then Cucumis melo. These crude lipid mixtures were used to prepare liposomes from each extract: Canna indica (LCI), Cucumis melo (LCM) and Prunus armeniaca (LPA). The liposome optical density, susceptibility to oxidation degradation and interaction with human WBCs was then evaluated.
Figure 1 shows the optical densities of the liposomes incubated for up to 28 days at RT. The differences in optical densities seen for LCI (Fig. 1b), LCM (Fig. 1c) and LPA (Fig. 1d) compared to PC liposomes (Fig. 1a) may be due to the different compounds within each sample. Liposome aggregation as a function of time was followed by monitoring liposome suspension turbidity at 400 nm (Fig. 1), which showed changes in aggregation from 0 to 28 days of 26.6, 11.8 and 6.1% for LCI, LCM and LPA, respectively. These values were lower than those seen for PC liposomes (43%). The aggregation tendency depends on the surface properties of liposomes, which is may be changed by the peroxidation of constituting lipids.
LCI, LCM and LPA were less susceptible to oxidation during the incubation period (28 days) than PC liposomes (Table 2). It was observed that all the formed liposomes have different activity against lipid peroxidation. This peroxidation inhibition of the LPA liposomes (71.8%) was higher than that of LCI (52.7%) and LCM (34.5%), while PC liposomes (17.6%) was the least. This difference may be due to the presence of different compounds in the liposomes formed from the acetone extracts. Since phospholipids in liposomes are susceptible to oxidation during incubation, the presence of antioxidant molecules (polyphenolic compounds) such as ARs, xanthones, flavonoids and others (Yen and Chuang, 2000) could minimize the oxidation degradation of liposomes during the incubation period at room temperature and could be potentially responsible for the powerful activity of the extracts and the main factor for the protection of the formed liposomes from peroxidation compared to the PC liposomes. The difference in the inhibition of peroxidation may be due to the type and concentration of the antioxidant compounds in the acetone extract.
To assess the antioxidant properties of the molecules present in the extracts, LCI, LCM and LPA were incubated with human WBCs. The scavenging activity of the extract liposomes was measured in terms of their capacity to inhibit free radical generation. Free radicals are molecules or molecule fragments with unpaired electrons in the outer orbital (Sanchez-Moreno, 2002), which renders them very reactive and capable of initiating chain reactions that result in oxidation degradation of lipids and tissue damage.
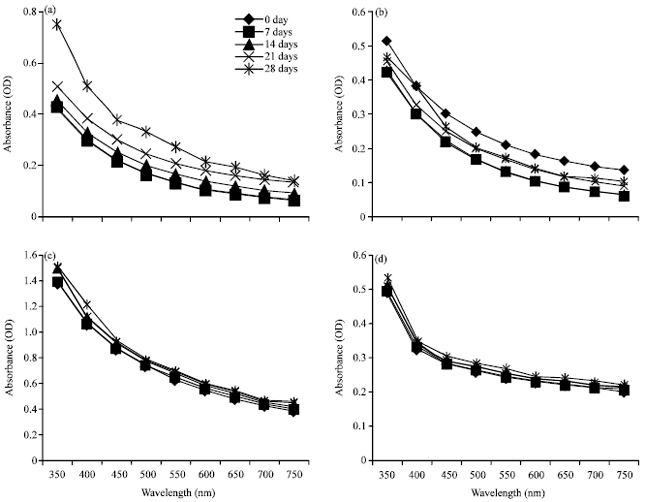 | |
| Fig. 1(a-d): | Time course (days) of different liposome optical densities, (a) Phosphatidylcholine liposomes (PC), Liposomes formed from crude acetone extract of, (b) Canna indica (LCI), (c) Cucumis melo (LCM) and (d) Prunus armeniaca (LPA) |
| Table 2: | Percent inhibition of lipid peroxidation in LCI, LCM and LPA liposomes compared to PC liposomes (control) as a function of time |
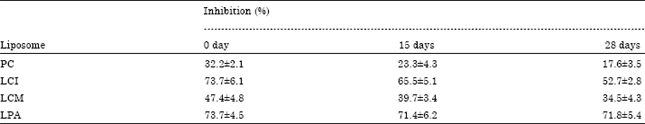 | |
| Data represented as Mean±SD, Values are statistically significant at p<0.05 | |
Free radicals are produced via metabolic pathways and phagocytosis of some WBCs. One free radical is O2-, which can form oxidants that are toxic towards phagocytes. O2- reduces NBT into Formazan, which can be spectrophotometrically measured at 570 nm (Sanchez-Moreno, 2002).
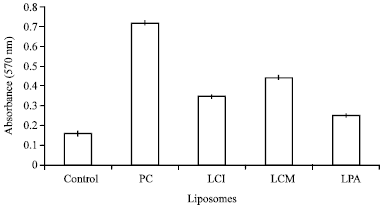 | |
| Fig. 2: | Effect of incubating different liposomes with human white blood cells on O2- radical production compared to control (without liposomes). Mean of triplicate±SD, p<0.05 |
Liposomes formed from the acetone extracts were found to have the ability to scavenge free radical species (O2-), which indicates that they have antioxidant power to inhibit O2- production (Fig. 2). In general, control cells themselves produced free radicals (0.15) but the presence of liposomes enhanced the production of O2- in these cells to 0.7 for PC liposomes and 0.34, 0.45 and 0.25 for LCI, LCM and LPA, respectively. These results indicate that the antioxidant activity of the additional molecules present in the extract liposomes enhanced their scavenging activity towards free radicals by reducing O2- production and subsequently inhibited Formazan production by 38% (LCM), 52% (LCI) and 65% (LPA) as compared to PC liposomes.
This experiment demonstrated that liposomes can influence certain functional responses of human neutrophils in vitro to produce superoxide ions. The increase was composition dependent, indicating that the extract liposomes (LCI, LCM and LPA) may contain different compounds (especially antioxidant molecules) that resulted in reduction of superoxide ions formation compared to PC liposomes. Our qualitative experiments (TLC) on these extracts revealed the presence of different molecules, including ARs, which exhibit antioxidant activity and are powerful free radical scavengers.
In general, present results indicated that the molecular mixture obtained from these extracts can be used to form liposomes that have high stability as determined by the decrease in liposome aggregation (as measured by changes in turbidity), minimal oxidative degradation of lipids and a capacity to reduce free radical production. These features would be especially useful in systems using liposomes as vehicles for drug encapsulation or as a delivery system for several types of molecules.
Liposomes prepared from Canna indica, Cucumis melo and Prunus armeniaca extracts exhibited high antioxidative property and could minimize lipid oxidation. Their interaction with human WBCs resulted in reduced amounts of free radical formation, indicating that these liposomes are both stable and exhibit antioxidant effects that reduce the production of free radicals by virtue of their lipid composition and in particular the presence of alkylresorcinols. Further studies are needed to analyze all the compounds present in these extracts and to develop these liposomes as a model for biological and drug systems for use in vitro and in vivo.
This study was supported by the Deanship of the Academic Research (Grant No. 792/14/120) at Mutah University. The technical assistance of Abdulla Allawneh is greatly acknowledged.