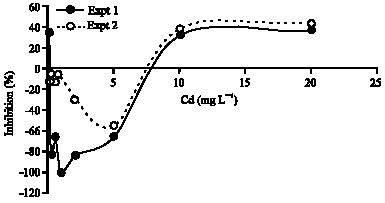
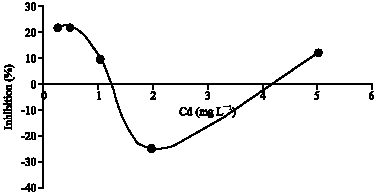
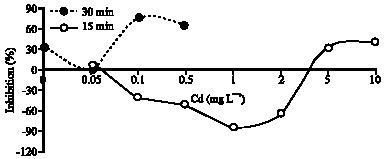
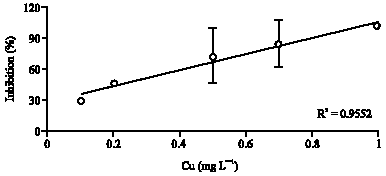
Research Article
Toxicity Biosensor for the Evaluation of Cadmium Toxicity Based on Photosynthetic Behavior of Cyanobacteria Anabaena torulosa
Faculty of Science and Technology, University Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Salmijah Surif
Faculty of Science and Technology, University Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Lee Yook Heng
Faculty of Science and Technology, University Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia