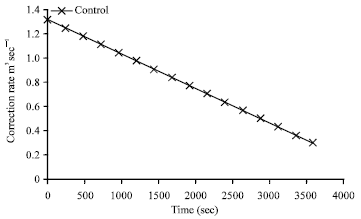
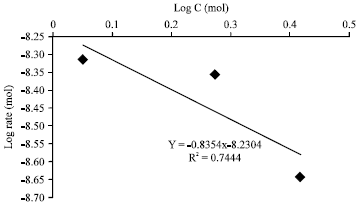
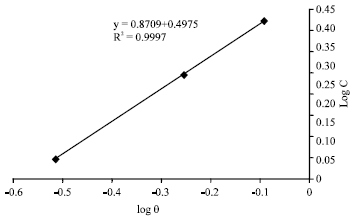
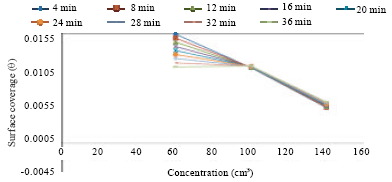
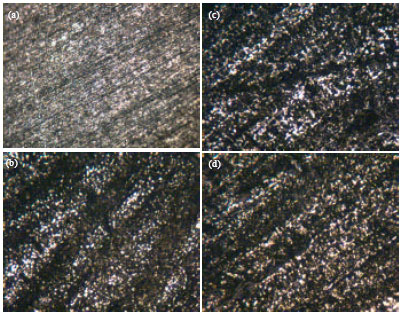
Research Article
Evaluating the Deterioration Behaviour of Mild Steel in 2 M Sulphuric Acid in the Presence of Butyrospermum parkii
Deparment of Mechanical Engineering, Covenant University, PMB 1023, Ota, Nigeria
O.O. Ajayi
Deparment of Mechanical Engineering, Covenant University, PMB 1023, Ota, Nigeria
O. Fayomi
Deparment of Mechanical Engineering, Covenant University, PMB 1023, Ota, Nigeria
V.O. Ifepe
Deparment of Mechanical Engineering, Covenant University, PMB 1023, Ota, Nigeria