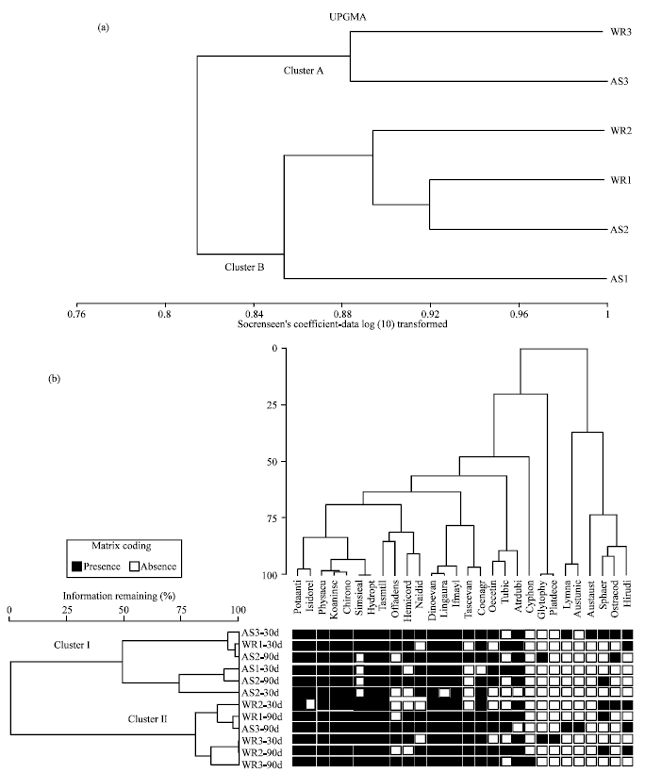
Research Article
Impact of Exotic Willow Roots (Salix spp.) as Habitat for Aquatic Invertebrate Communities in South Australian Stream
Department of Biological Sciences, Faculty of Science and Technology, Universiti Malaysia Terengganu, 21030 Kuala Terengganu, Terengganu, Malaysia
J.T. Jennings
School of Earth and Environmental Sciences, The University of Adelaide, South Australia 5005, Australia