Research Article
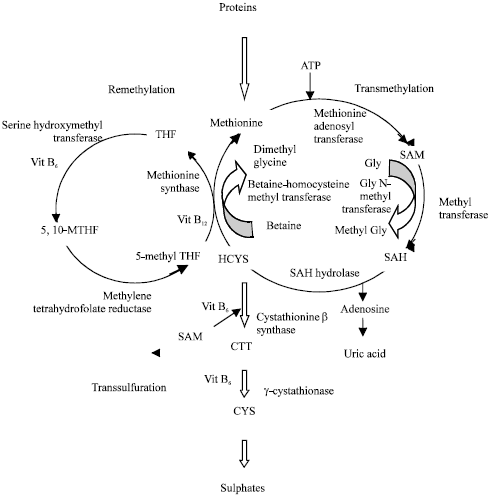
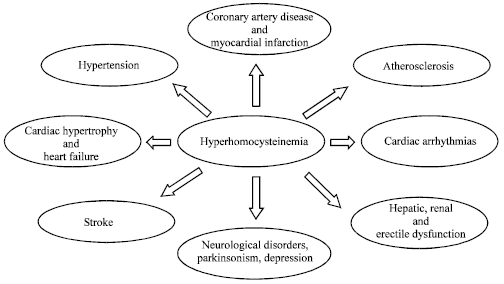
Hyperhomocysteinemia and Cardiovascular Disorders: Is There a Correlation?
I.S.F. Institute of Pharmaceutical Sciences and Drug Research, Moga, Punjab, India
Amrit Pal Singh
Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India
Subrahmanya S. Ganti
I.S.F. Institute of Pharmaceutical Sciences and Drug Research, Moga, Punjab, India
Manjeet Singh
I.S.F. Institute of Pharmaceutical Sciences and Drug Research, Moga, Punjab, India