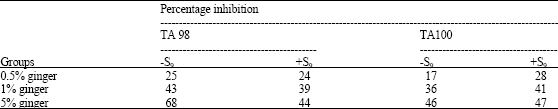
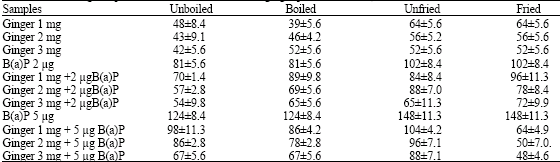
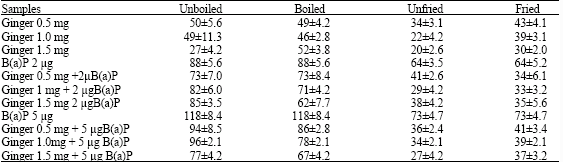
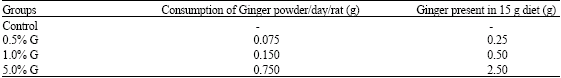Research Article
In vivo Antimutagenic Potential of Ginger on Formation and Excretion of Urinary Mutagens in Rats
Food and Drug Toxicology Research Centre, National Institute of Nutrition (ICMR) PO-Jamai Osmania, Hyderabad-500 007, India
T. Prasanna Krishna
Food and Drug Toxicology Research Centre, National Institute of Nutrition (ICMR) PO-Jamai Osmania, Hyderabad-500 007, India
K. Polasa
Food and Drug Toxicology Research Centre, National Institute of Nutrition (ICMR) PO-Jamai Osmania, Hyderabad-500 007, India