Research Article
Efficient Plant Regeneration Through Somatic Embryogenesis from Leaf Base-derived Callus of Kaempferia galanga L.
Not Available
M.N. Amin
Not Available
T. Ahamed
Not Available
M. R. Ali
Not Available
A. Habib
Not Available
Kaempferia galanga L. (vemac. Black Thorn in English and Chandramul in Bengali) belonging to the family Zingiberaceae is an aromatic perennial herb with tuberous rootstocks. The plant is native to India, but currently it is cultivated mainly in South East Asia and China[1]. In Bangladesh the plant is specially grown in Sal forest in Dhaka and Maymensingh[2]. Kaempferia galanga is an important medicinal plant. The rhizomes of the plant are widely used in East Asia for a wide range of medicinal applications[3]. Its rhizome contains a volatile oil[4] and several alkaloids, starch, protein, amino acid, minerals and fatty matter. Leaves and flowers contain flavonoids[2]. Rhizomes are aromatic, stimulant, carminative, diuretic and stomachic. They are also used for coughs, pectoral affections and stoppage of the nasal blocks, asthma and hypertension. In the form of lotions and poultices leaves are applied to sore eyes, sore throat, rheumatism, swellings and fibers. For its aroma and flavour they are also used in flavouring foodstuffs, beverages, perfume and cosmetics industries.
In conventional method, Black thorn is propagated vegetatively by the stored rhizomes but disease susceptibility and higher cost of production have restricted its cultivation. In the recent years micropropagation techniques are being profitably used to overcome such constraints in various crops including ornamental and horticultural plants. Due to considering the present demand, (both for economic and medicinal values) and propagation problem of the plant, it is necessary to develop a suitable protocol for mass propagation from existing elite cultivars. The process of somatic embryogenesis is a suitable method of micropropagation and has the potential for mass propagation commercially at low cost per unit. There are a few reports on in vitro culture of some rhizomatous plants like ginger[5-7], cardamom[8] and Alphinia calcarata[9]. The potential use of tissue culture technique in Kaempferia galanga L. was demonstrated by Vincent et al.[10-11] who reported complete plantlet regeneration through callus culture and somatic embryogenesis.
The present study was conducted to establish a suitable plantlet regeneration protocol for Kaempferia galanga using in vitro techniques. This is perhaps the first report on in vitro plant regeneration of Kaempferia galanga L. in Bangladesh. The results may be of some importance as pioneering study on tissue culture of this aromatic medicinal plant.
Leaf base segments were excised aseptically from in vitro grown cultures, which were originally established from the rhizome buds of field grown mature plants. The sheathing basal parts of excised leaves were chopped into pieces (1-1.5 mm) and segments were placed on the agar-gelled medium. The leaf base explants were ultimately cultured on MS medium[12] supplemented with various concentrations of 2, 4-D alone and in combination with BA for callus induction. Seven weeks old callus was thus subcultured on MS medium containing different combinations of BA with NAA or IBA for somatic embryogenesis.
The embryogenic calluses were subcultured at monthly intervals on same medium for somatic embryos formation and development of complete plantlets with shoots and roots.
The pH of the culture media was adjusted to 5.7±0.1 before addition of agar and sterilized by autoclaving for 20 min at 1.1 kg cm-2 pressure at 121°C.
For solidifying the medium, 7-8 g L-1 agar or 2.0 g L-1 gelrite was used. The tubes or flasks containing explants were incubated on culture racks in the growth chamber. The cultures were maintained at 25±2°C under the cool white fluorescent lights for 16 h photoperiod with a photon flux density of about 70 μ mol m-2s-1.
Induction of callus: Excised in vitro leaf base segments were cultured on MS medium with various levels of 2, 4-D alone and in combination with BA for induction of callus. After six weeks of culture incubation callus mass increased to a transferable size (Fig. 1A). Morphogenic potentialities of the explant found to differ depending on the growth regulator supplements (Table 1).
Among different combinations of the auxin and cytokinin tested, 2, 4-D + BA was found highly effective for induction of callus (results of the other combinations are not shown in the Table 1). It was mostly soft, faster growing, light creamy-white and friable. The highest frequency of callus induction (85%) was recorded at the end of 7 weeks on medium containing 1.5 mg L-1 2, 4-D with 1.0 mg L-1 BA. Similar combination of auxin with cytokinin for callus induction has been reported in the past by Malamug[6] on ginger, Vincent et al.[11] on Kaempferia galanga, Muthukumar et al.[13] on Datura metel. Callus induction from leaf base explant has also been achieved in cardamom using 2, 4-D+ Kn in combination[8].
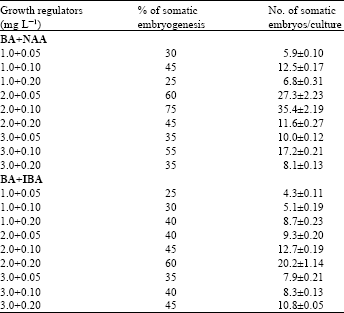
Embryogenesis from the subcultured callus: The callus tissues developed from leaf base explants were subcultured on MS medium with various concentrations of BA with NAA and BA with IBA for somatic embryogenesis (Table 2).
| Table 1: | Effects of different concentrations of 2, 4-D alone and in combination with BA for callus induction from leaf base explant. There were 20 explants in each treatment and data ( |
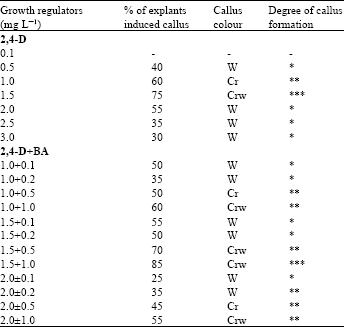 | |
| (-) Indicate no response; (*) slight callusing; (**) considerable callusing and (***) profuse callusing; Cr = Creamy; CrW = Creamy-white; W = White | |
| Table 2: | Effects of different concentrations and combinations of BA with NAA or IBA for somatic emtayogenesis on MS medium from leaf base derived callus. There were 20 cultures in each treatment and data (x±SE) were collected after 6 week of culture |
 | |
After three weeks of subculturing creamy white sectors with well-developed embryoids were seen that later turned into green shoots (Fig. 1B). Although root initiation started from these calli after two weeks of subculturing but complete plants were seen in the differentiating callus after three weeks (Fig. 1C) and a single leaf to multiple shoots were observed after five weeks of culture (Fig. ID).
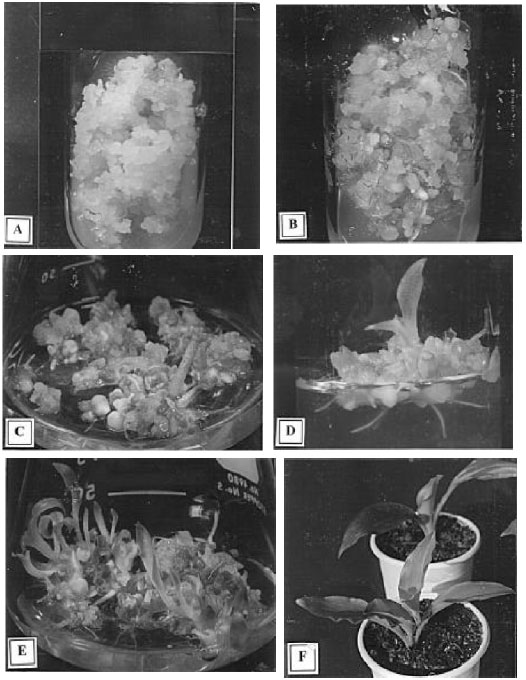 | |
| Fig. 1: A-F: | Plantlets regenatation from the leaf base explant through somatic embryogenesis of Kaempferia galcmga L. |
| A: | Embiyogenic callus formation from the leaf base explant on MS+1.5 mg L-1 2,4-D+1.0 mg L-1 BA after six weeks of culture | |
| B: | Development of embryoids from embryogenic callus on MS+2.0 mg L-1 BA+0.1 mg L-1 NAA whithin three weeks of subculture | |
| C: | shoots and roots development and elongation of plants on MS+2.0 mg L-1 BA+0.1 mg L-1 NAA whithin five weeks of subculture | |
| D: | shoots and roots development and elongation of plants on MS+2.0 mg L-1 BA+0.1 mg L-1 NAA whithin weight weeks of subculture | |
| E: | Complete plantlets with shoots and roots regenaration on same medium whithin ten weeks of subculture | |
| F: | Establishment of in vitro grown plantlets under ex vitro cnndition |
The maximum percentage of embryogenic callus that formed embryos was 75% at the BA+NAA concentration of 2.0 mg L-1+ 0.1 mg L-1. The highest number of somatic embryos per culture was 35.4±2.19, which was also recorded in the same concentration of BA+NAA after ten weeks of culture (Fig. 1E). Complete plant regeneration by embryogenesis was observed on different explants cultures of garlic[14-15], ginger[5], Muntmgia calabura[16]. Using BA+NAA combinations adventitious shoot regeneration from leaf base explant has been observed on cardamom[8]. Qrganogenesis and plant formation in presence of BA or Kn with NAA was also reported from various explants via callus culture of ginger[6], Kaempferia galanga[10] and sugarcane[17] .
Plantlets were at first established in small plastic pots with a view to easy handling and maintenance during acclimatization and transplantation. About 95% of the plantlets established under ex vitro condition when they were initially transferred on coco-peat as potting mix. On the other hand, 90% survival rate of the plantlets was observed when they were transferred on ice cream pots containing garden soil, compost and sand (2:2:1). It was also observed 85% of the plantlets could be established under ex vitro conditions when they were transferred on earthen pots containing soil and organic manure (Fig. 1F).