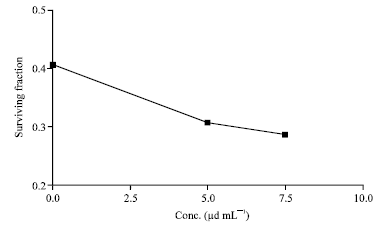
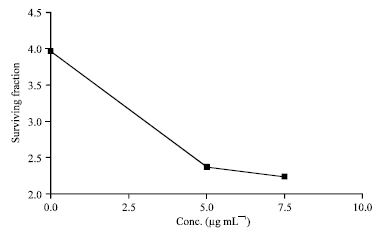
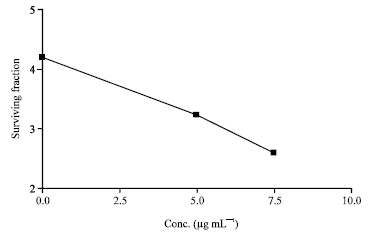
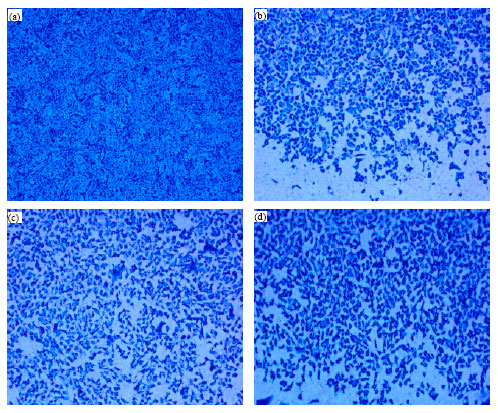
Research Article
The Cytotoxic Effect of PM 701 and its Fractions on Cell Proliferation of Breast Cancer Cells, MCF7
Department of Biology, Faculty of Science, King Abdul Aziz University (KAU), P.O. Box 80216, Jeddah 21589, Kingdom of Saudi Arabia
LiveDNA: 966.3097
ORCID: 0000-0002-7298-6548