Research Article
Advances in Micropropagation of Selected Aromatic Plants: A Review on Vanilla and Strawberry
Department of Biotechnology, Instrumentation and Environmental Science, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, W.B., India
LiveDNA: 91.241
ORCID: 0000-0001-5059-2428
N. Mandal
Department of Biotechnology, Instrumentation and Environmental Science, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, W.B., India
S. Nandy
Crop Quality Control, Regina, Saskatchewan, Canada









Mukundraj Govindrao Rathod Reply
This research paper is very much interesting and i am very greatful by knowing this information.
keep it up bye
Mukundraj Govindrao Rathod
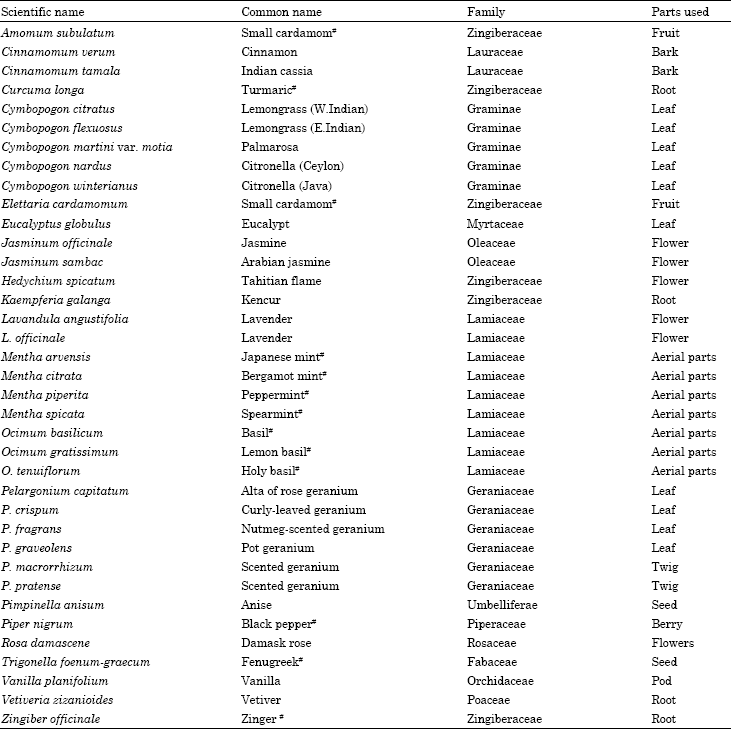
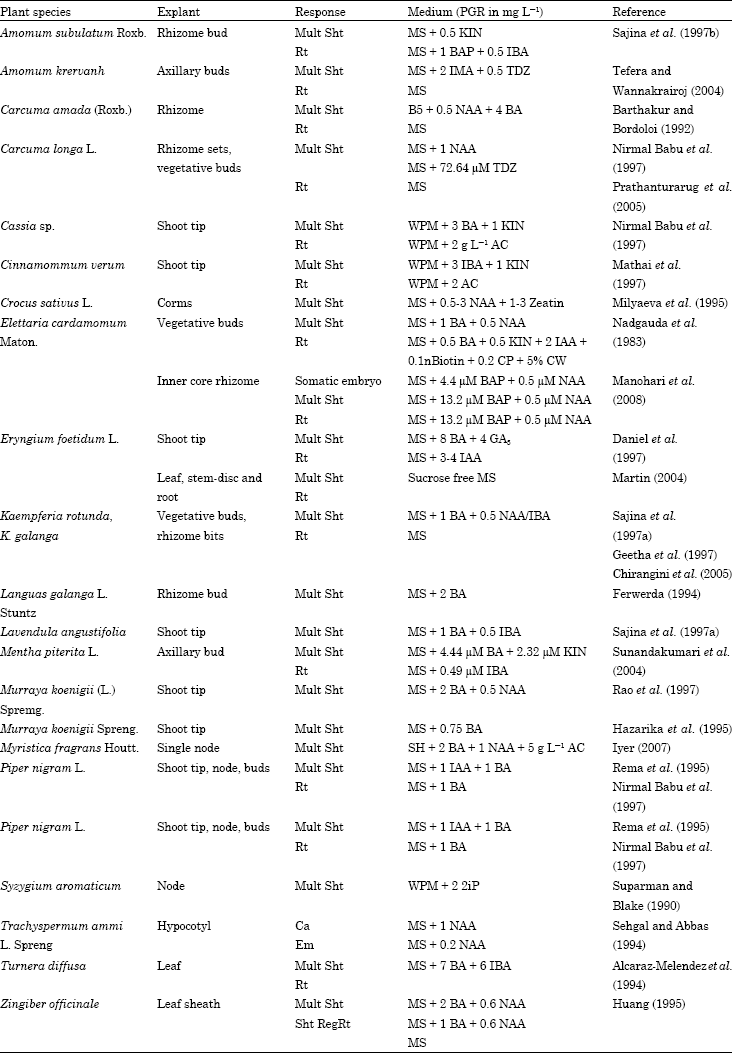
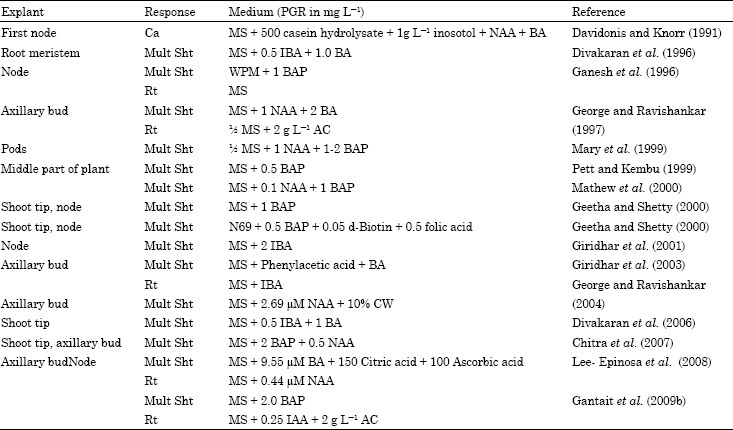
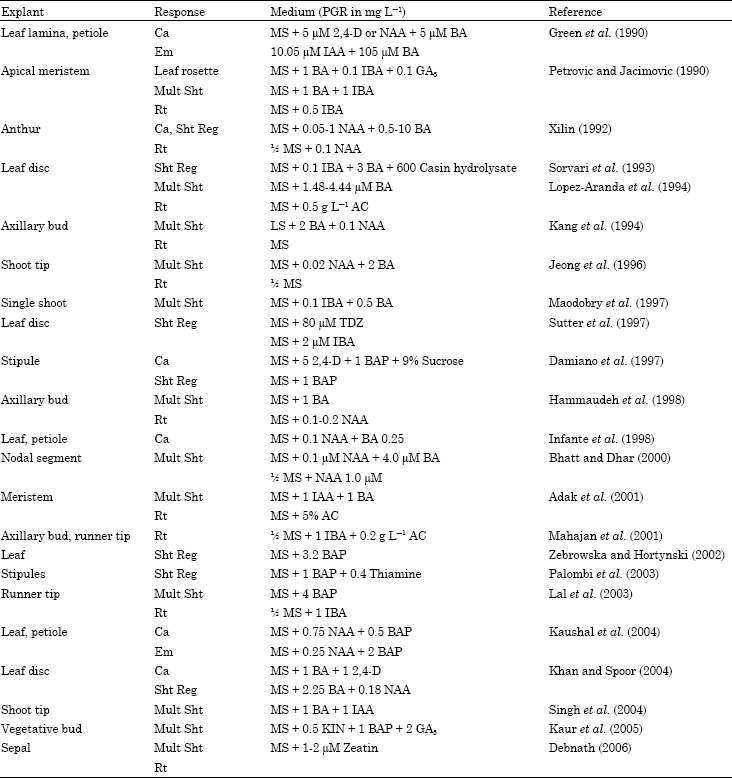
Selected explants are commercially important.