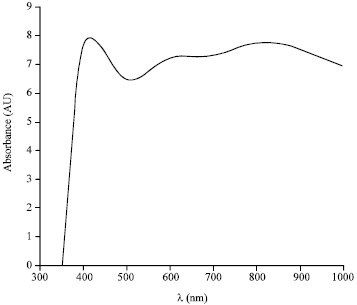
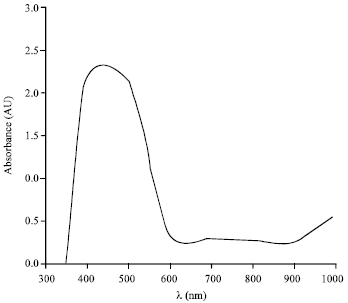
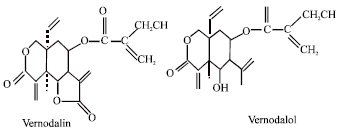
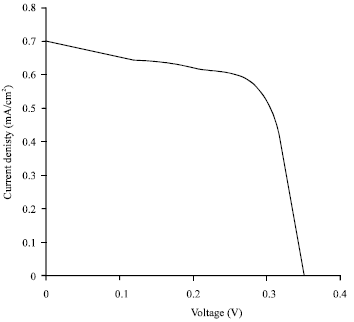
Research Article
Bitter Leaf (Vernonia amygdalin) for Dye Sensitized Solar Cell
Department of Physics, Lagos State University, Ojo, Badagry, Nigeria
M.B.O. Shitta
Department of Physics, Ekiti State University of AdoEkiti, Nigeria
T. Oluwa
Department of Botany, Lagos State University, Ojo, Badagry, Nigeria
S. Adeola
Department of Biochemistry, Lagos State University, Ojo, Nigeria