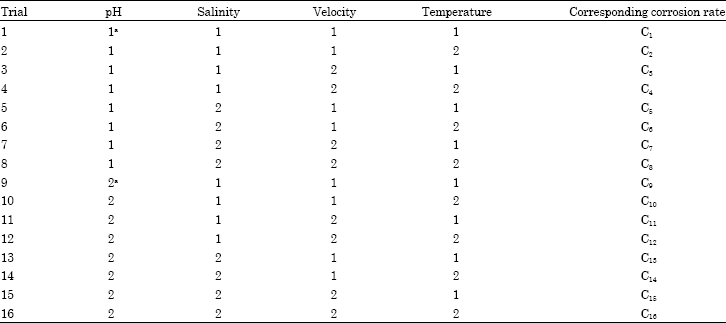
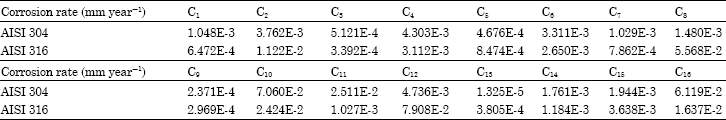
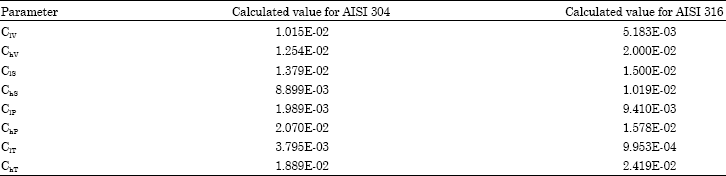
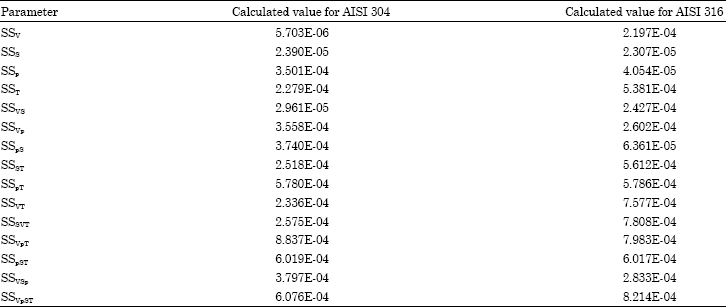
Research Article
Comparative Studying of Marine Parameters’ Effect, via Quantitative Method
Department of Materials Science and Engineering, Shiraz University, Shiraz, Iran
M. Pakshir
Department of Materials Science and Engineering, Shiraz University, Shiraz, Iran
A. Yazdani
Department of Materials Science and Engineering, Shiraz University, Shiraz, Iran