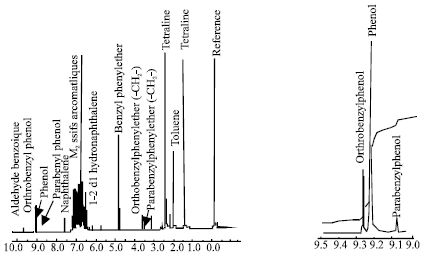
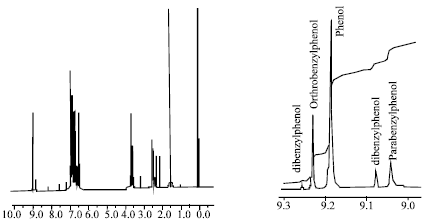
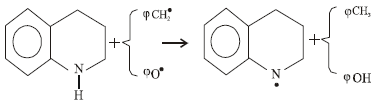
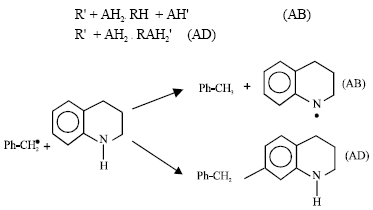
Research Article
Ranking of Hydrogen Donor Reactivity by Model Acceptor and by Coal Conversion
Department of Chemistry University of Benin, Benin City, Nigeria
D. Nicole
Laboratoire dEtude des solutions organiques et colloidales, UA CNRS 406, BP 239, 54506 Vandoeuvre-Les- Nancy Cedex, France