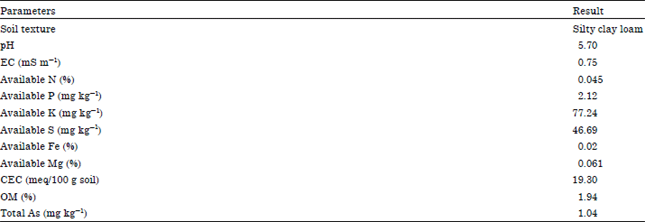
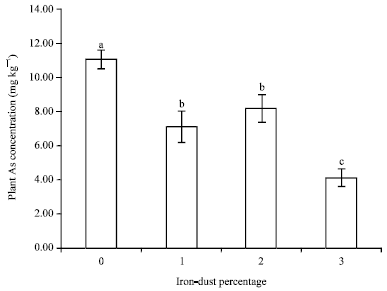
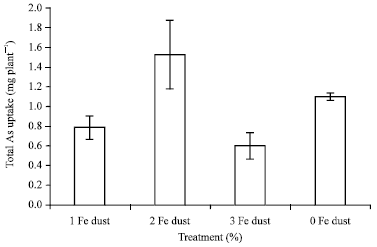
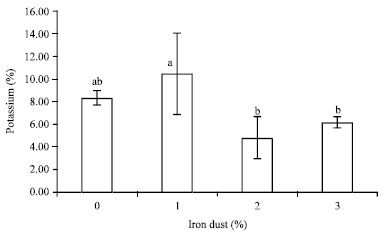
Research Article
Effect of Iron Dust on Arsenic Accumulation and Nutrient Status of Ipomoea aquatica Grown in Arsenic Contaminated Soil
Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong 4220, Chittagong, Bangladesh
Abu Sayeed Mohammad Mahmud
Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong 4220, Chittagong, Bangladesh
Md. Sadiqul Amin
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Sheikh Mohammad Fazle Rabbi
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Faridul Islam
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Md. Saidur Rahman
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Nemai Chandra Nandi
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Nusrat Jahan Mouri
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Sunuram Ray
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh
Jagadish Chandra Joardar
Soil Science Discipline, Khulna University, Khulna-9208, Khulna, Bangladesh