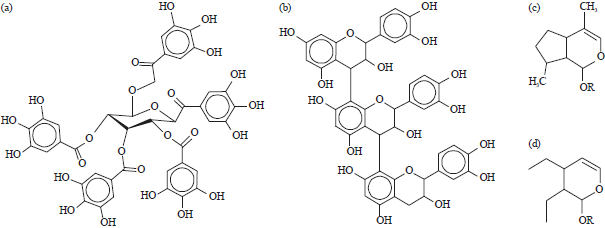
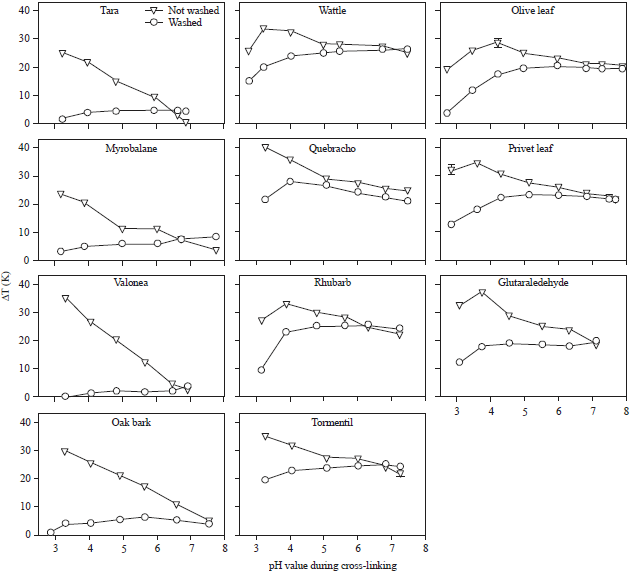
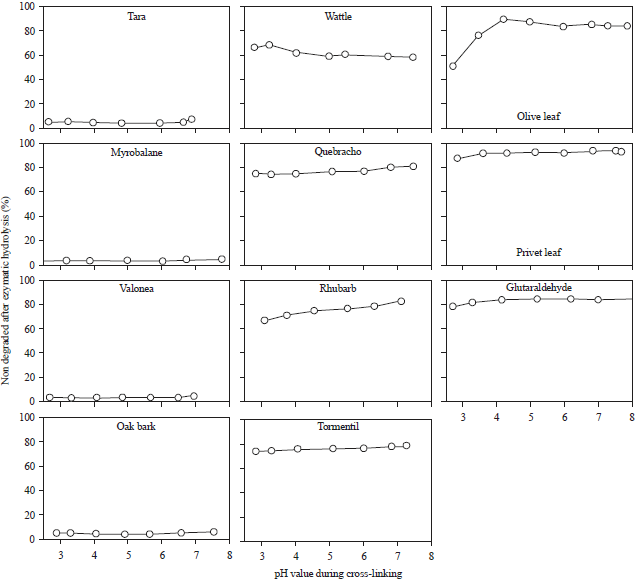
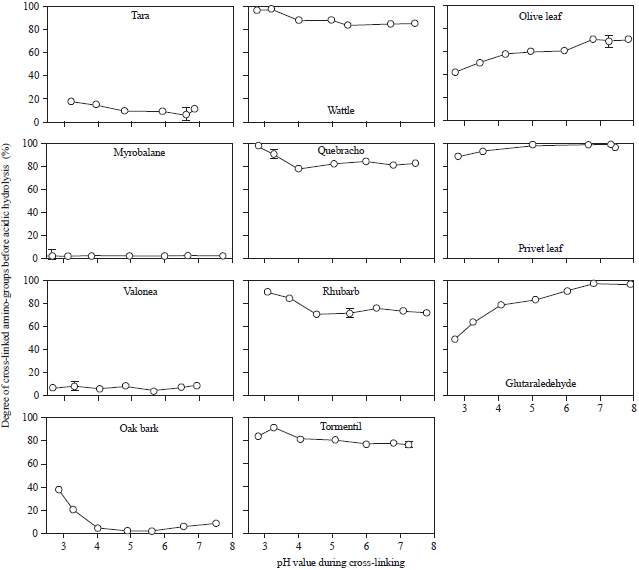
Research Article
Investigations Towards the Binding Mechanisms of Vegetable Tanning Agents to Collagen
Research Institute of Leather and Plastic Sheeting, Freiberg, Germany, Meissner Ring 1-5, 09599 Freiberg, Germany
Michael Meyer
Research Institute of Leather and Plastic Sheeting, Freiberg, Germany, Meissner Ring 1-5, 09599 Freiberg, Germany