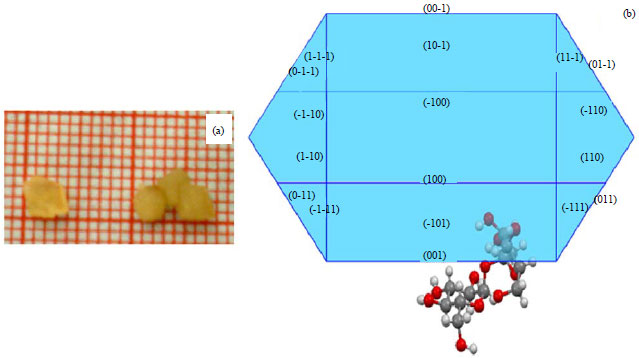
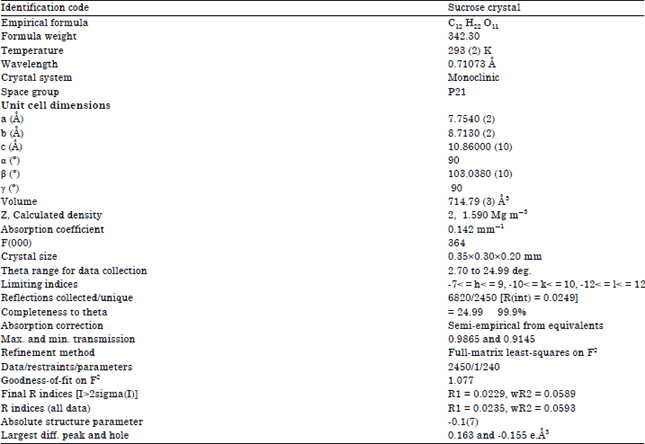
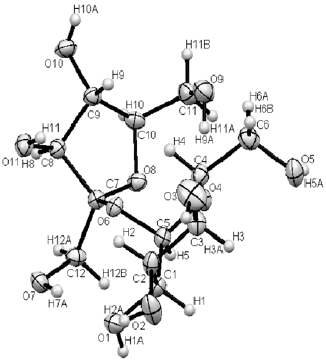
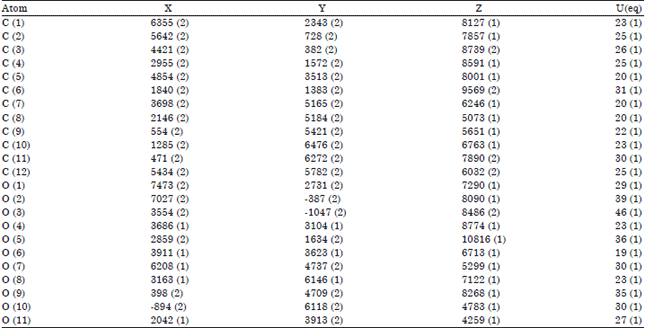
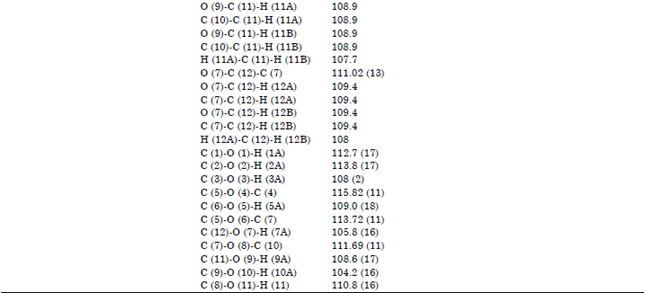
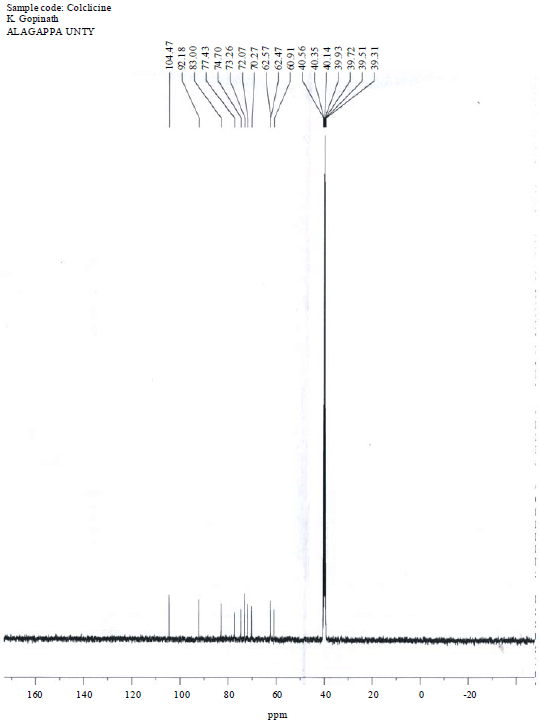
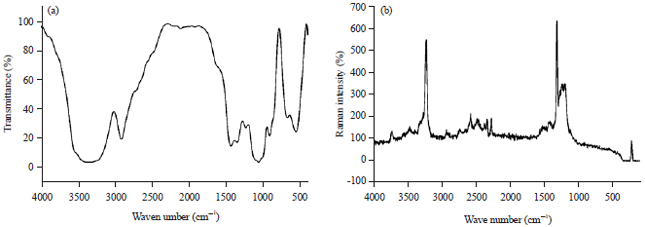
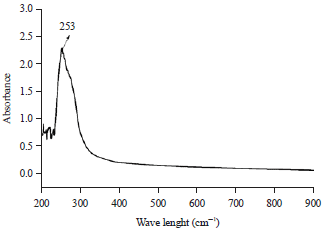
Research Article
Phytochemical Synthesis and Crystallization of Sucrose from the Extract of Gloriosa superba
Department of Nanoscience and Technology, Alagappa University, Karaikudi, 630003, Tamil Nadu, India
C. Karthikeyan
PG and Research Department of Physics, Jamal Mohamed College, Tiruchirappalli, 620020, Tamil Nadu, India
A.S. Haja Hameed
PG and Research Department of Physics, Jamal Mohamed College, Tiruchirappalli, 620020, Tamil Nadu, India
K. Arunkumar
PG and Research Department of Botany, Alagappa Government Arts and Science Collage, Alagappa Univesity, Karakudi, 630003, Tamil Nadu, India
A. Arumugam
Department of Nanoscience and Technology, Alagappa University, Karaikudi, 630003, Tamil Nadu, India