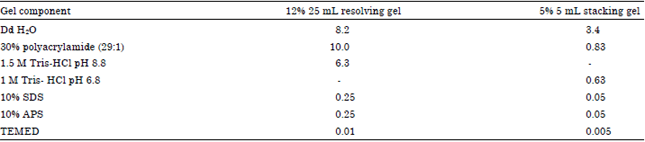
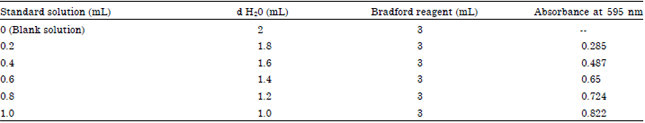
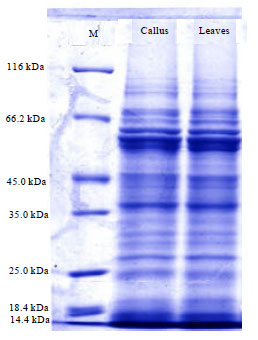
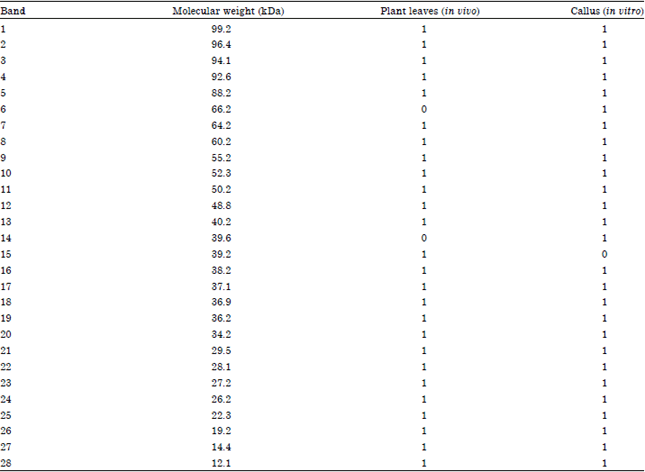
Research Article
In vivo and in vitro Variation in Protein Profiling in Withania somnifera (L.) Dunal
Laboratory of Plant Physiology and Biochemistry, Department of Botany, University of Rajasthan, Jaipur, Rajasthan, India
Richa Bhardwaj
Laboratory of Plant Physiology and Biochemistry, Department of Botany, University of Rajasthan, Jaipur, Rajasthan, India
Ankita Yadav
Laboratory of Plant Physiology and Biochemistry, Department of Botany, University of Rajasthan, Jaipur, Rajasthan, India
R.A. Sharma
Laboratory of Plant Physiology and Biochemistry, Department of Botany, University of Rajasthan, Jaipur, Rajasthan, India