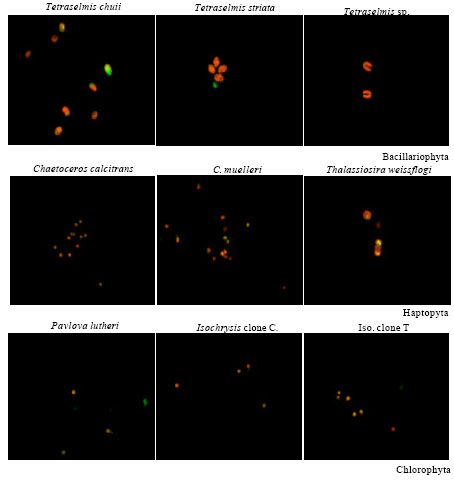
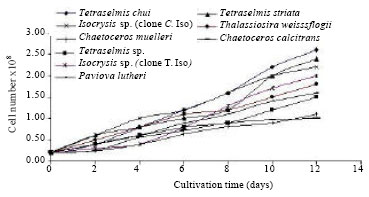
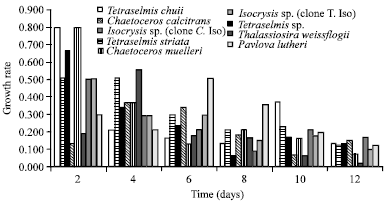
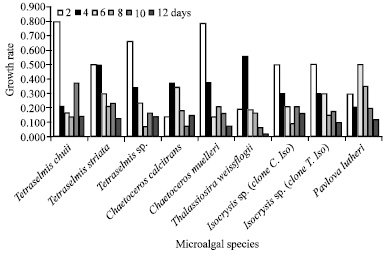
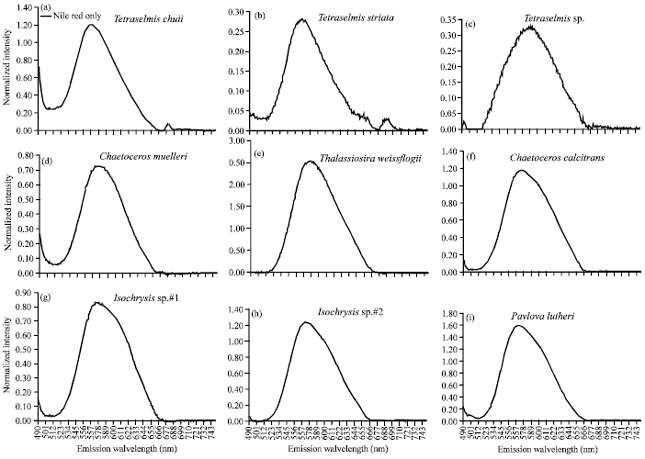
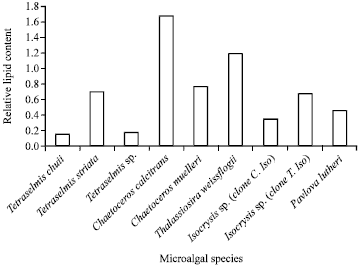
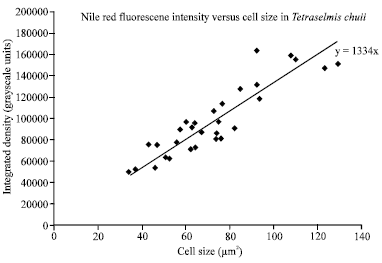
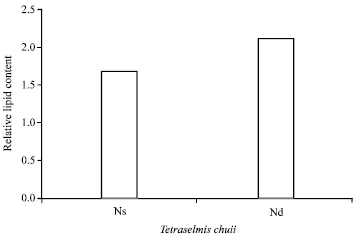
Research Article
Age of Nitrogen Deficient Microalgal Cells is a Key Factor for Maximizing Lipid Content
Department of Botany and Microbiology, Faculty of Science, Alexandria University, Egypt
Christopher W. Rieken
Josephine Bay Paul Center, Marine Biological Laboratory, Woods Hole, MA, USA
Scott R. Lindell
Marine Resource Center, Marine Biological Laboratory, Woods Hole, MA, USA
Christopher M. Reddy
Department of Marine Chemistry and Geochemistry, Oceanographic Institute, Woods Hole, MA, USA
Hala M. Taha
Department of Botany and Microbiology, Faculty of Science, Alexandria University, Egypt
Connie Pui Ling Lau
Marine Resource Center, Marine Biological Laboratory, Woods Hole, MA, USA
Catherine A. Carmichael
Department of Marine Chemistry and Geochemistry, Oceanographic Institute, Woods Hole, MA, USA