Research Article
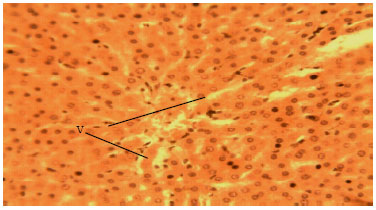
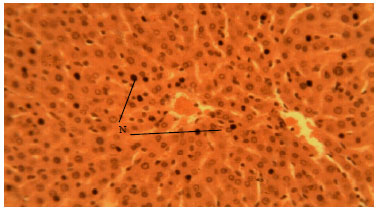
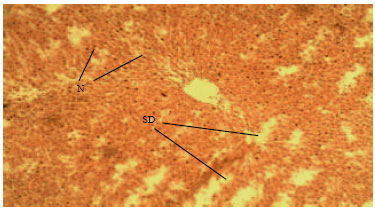
Methanolic Root Extract of Olax viridis Protects the Liver against Acetaminophen-induced Liver Damage
Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria
I.I. Madubunyi
Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria
S.C. Udem
Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria
C.O. Nwaigwe
Department of Animal Health and Production, Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria