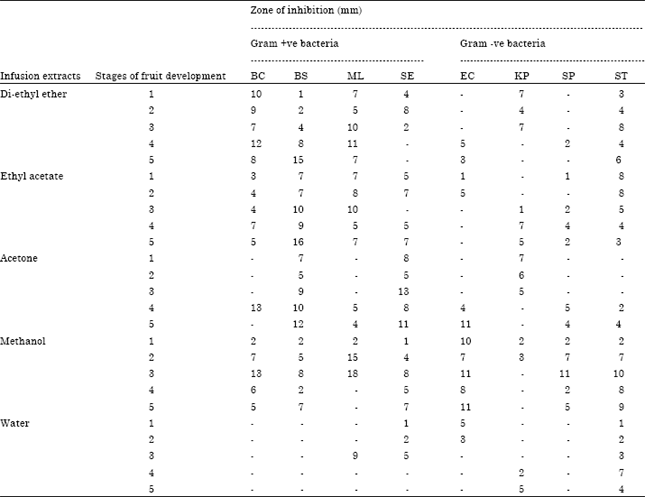
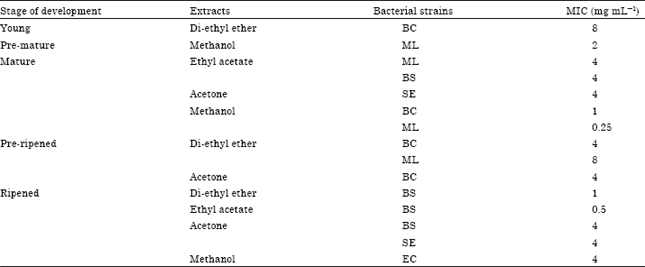
Research Article
Influence of Growth and Ripening of Physalis minima L. Fruit on its Antibacterial Potential
Plant Science Division, Agharkar Research Institute, G. G. Agarkar Road, Pune, Maharashtra-411 004, India
T.V. Ramana Rao
Plant Science Division, Agharkar Research Institute, G. G. Agarkar Road, Pune, Maharashtra-411 004, India