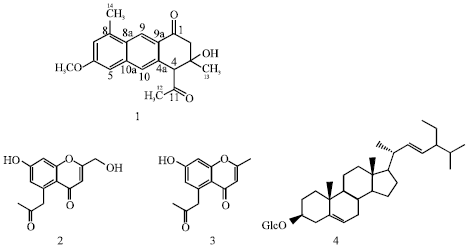
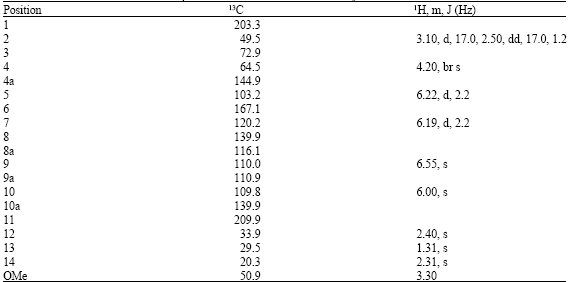
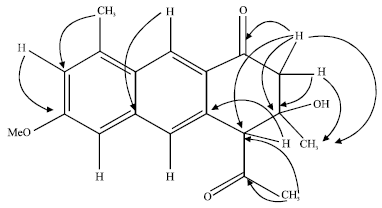
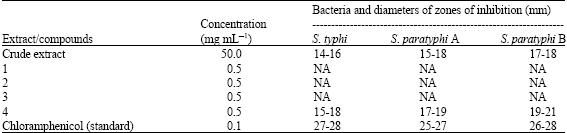
Research Article
An Antisalmonellal Agent and a New Dihydroanthracenone from Cassia petersiana
Department of Chemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Cameroon
Donatien Gatsing
Department of Biochemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Cameroon
Marguerite Kenmogne
Department of Chemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Cameroon
Dieudonne Ngamga
Department of Chemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Cameroon
Roseline Aliyu
Department of Biochemistry, Faculty of Medical Sciences, University of Jos, PMB 2084 Jos, Nigeria
Abiodun H. Adebayo
Department of Biological Sciences, College of Science and Technology, Covenant University, PMB 1023, Canaanland, Ota, Ogun State, Nigeria
Pierre Tane
Department of Chemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Cameroon
Bonaventure T. Ngadjui
Department of Organic Chemistry, Faculty of Science, University of Yaounde I, P.O. Box 812, Yaounde, Cameroon
Elisabeth Seguin
Laboratoire de Pharmacognosie, UFR de M6decine-Pharrnacie, 76183 Rouen Cedex, France
Godwin I. Adoga
Department of Biochemistry, Faculty of Medical Sciences, University of Jos, PMB 2084 Jos, Nigeria