Research Article
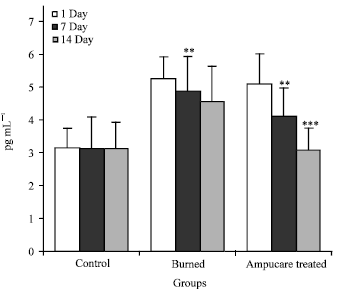
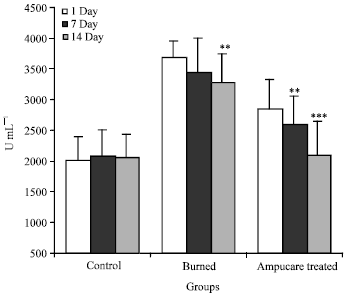
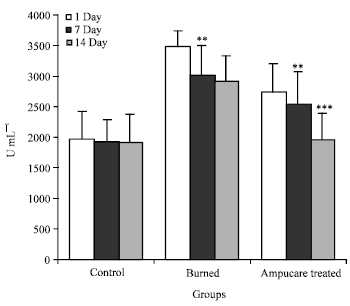
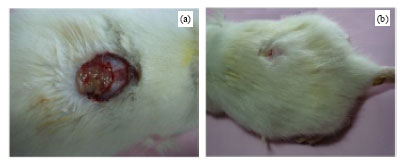
Plasma Cytokines and Trace Element Level in Severe Burn Rat Model-With Special Reference to Wound Healing Potential of Ampucare
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India
V.K. Dwivedi
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India
M. Chaudhary
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India
K. Malik
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India
V. Naithani
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India
S.M. Shrivastava
Pre-Clinical Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, H.P.-173205, India