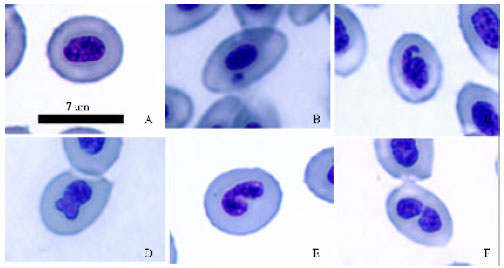
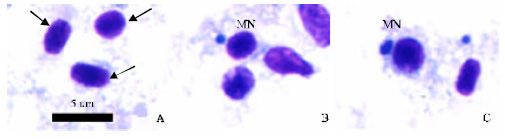
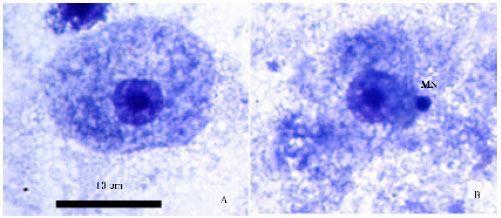
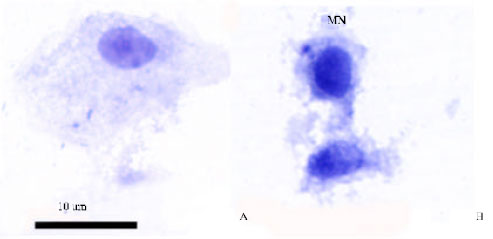
Research Article
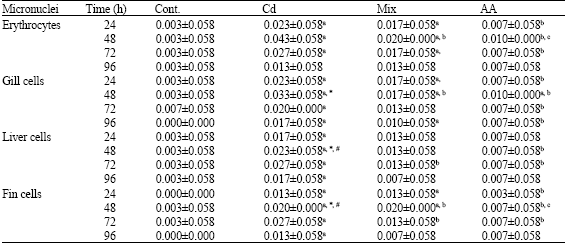
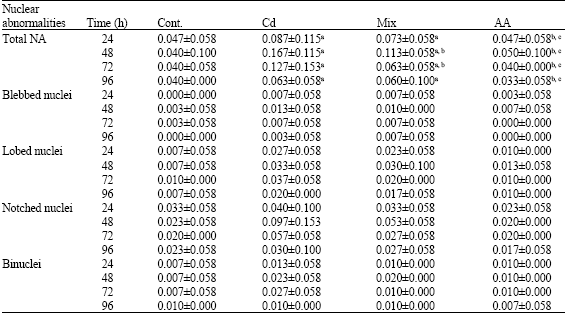
Micronucleus Test: The Effect of Ascorbic Acid on Cadmium Exposure in Fish (Puntius altus)
Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
Somphong Sahaphong
Mahidol University International College, Mahidol University, Salaya Campus, Nakhonpathom 73170, Thailand
Piya Kosai
Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
Myung-Huk Kim
Mahidol University International College, Mahidol University, Salaya Campus, Nakhonpathom 73170, Thailand