Research Article
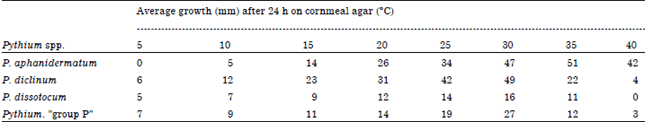
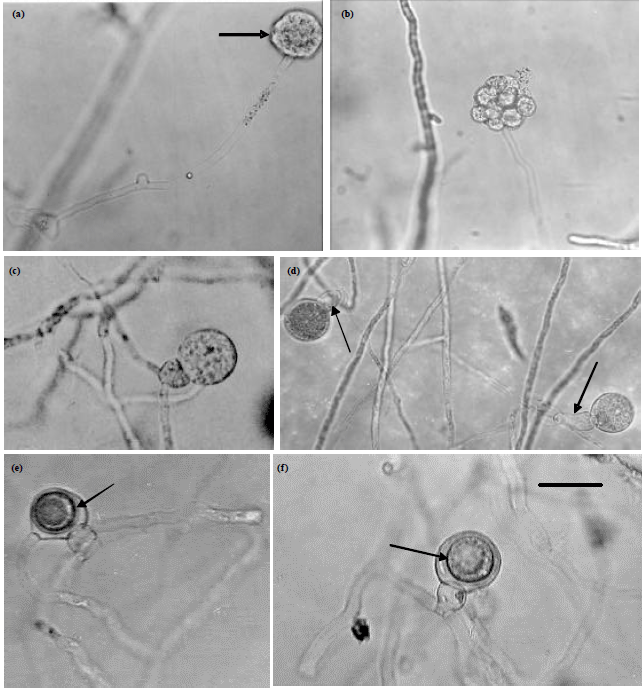
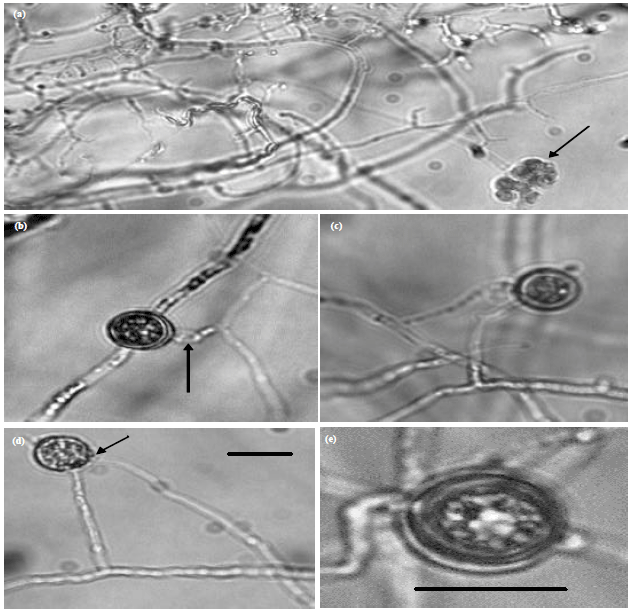
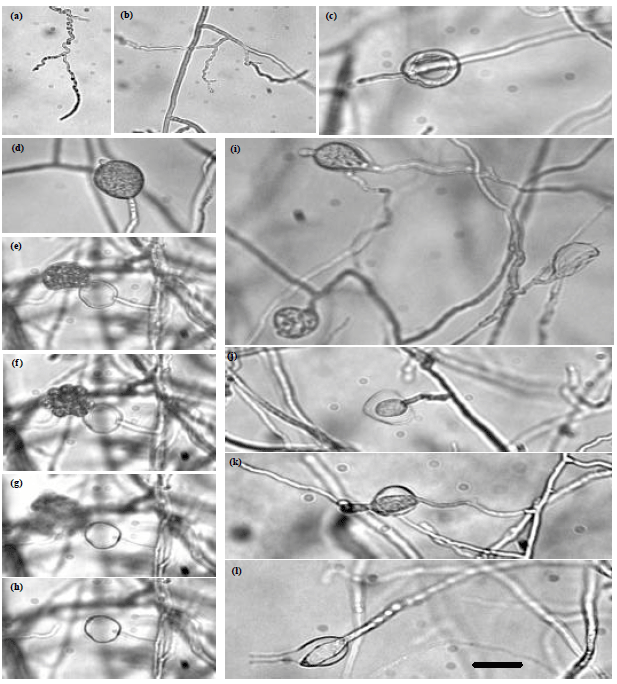
Occurrence, Identification and Pathogenicity of Pythium aphanidermatum, P. diclinum, P. dissotocum and Pythium "Group P" Isolated from Dawmat Al-Jandal Lake, Saudi Arabia
Department of Biology, College of Science, King Faisal University, AlHassa, Saudi Arabia
Hani M.A. Abdelzaher
Department of Biology, College of Science, Al-Jouf University, Sakaka, Saudi Arabia