Research Article
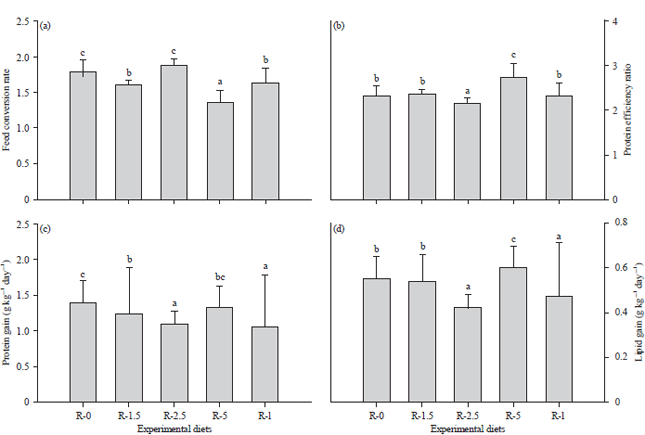
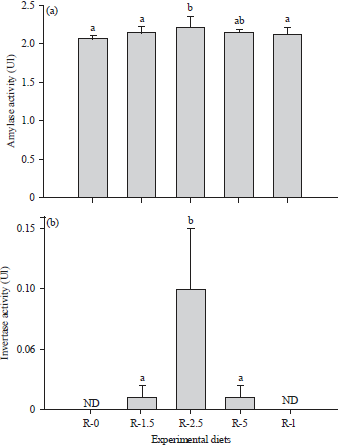
Effects of Carbohydrate-Rich Diets Containing Snail Digestive Juice on the Growth Performance and Glycosidase Activities of Nile Tilapia (Oreochromis niloticus)
Center for Research of Oceanology (CRO), Department of Aquaculture, P.O. Box V 18, Abidjan, Cote d’Ivoire
LiveDNA: 225.28791
Yeo Maurice Gopeyue
Laboratory of Biocatalysis and Bioprocesses, Faculty of Food Science and Technology, Nangui Abrogoua University, 02 P.O. Box 801 Abidjan 02, Cote d’Ivoire
Etchian Assoi Olivier
Laboratory of Biology and Animal Cytology, Faculty of Natural Science, Nangui Abrogoua University, 02 P.O. Box 801 Abidjan 02, Cote d’Ivoire
Yao Alla Laurent
Center for Research of Oceanology (CRO), Department of Aquaculture, P.O. Box V 18, Abidjan, Cote d’Ivoire
Dabonne Soumaila
Laboratory of Biocatalysis and Bioprocesses, Faculty of Food Science and Technology, Nangui Abrogoua University, 02 P.O. Box 801 Abidjan 02, Cote d’Ivoire