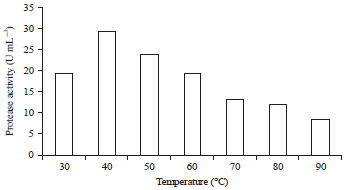
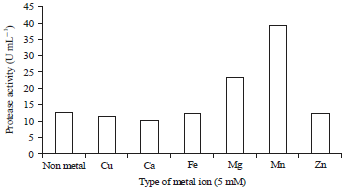
Research Article
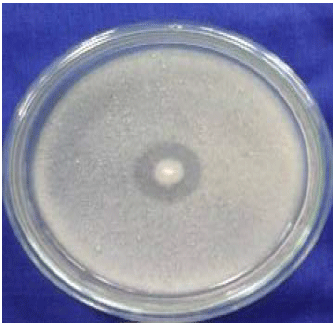
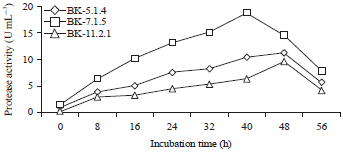
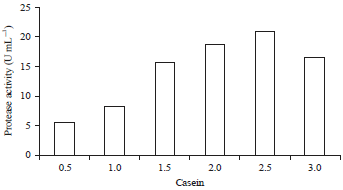
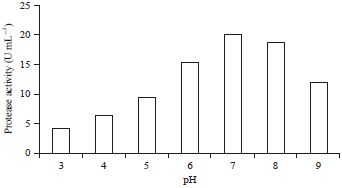
Isolation and Characterization of Lactic Acid Bacteria Proteases from Bekasam for use as a Beef Tenderizer
Department of Animal Production, Faculty of Animal Science, Jambi University, Indonesia
LiveDNA: 62.20625
Arnim
Department of Animal Production, Faculty of Animal Science, Andalas University, Padang, Indonesia
Yetti Marlida
Department of Animal Nutrition, Faculty of Animal Science, Andalas University, Padang, Indonesia
LiveDNA: 62.22962
Yuherman
Department of Animal Production, Faculty of Animal Science, Andalas University, Padang, Indonesia