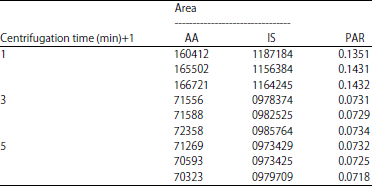
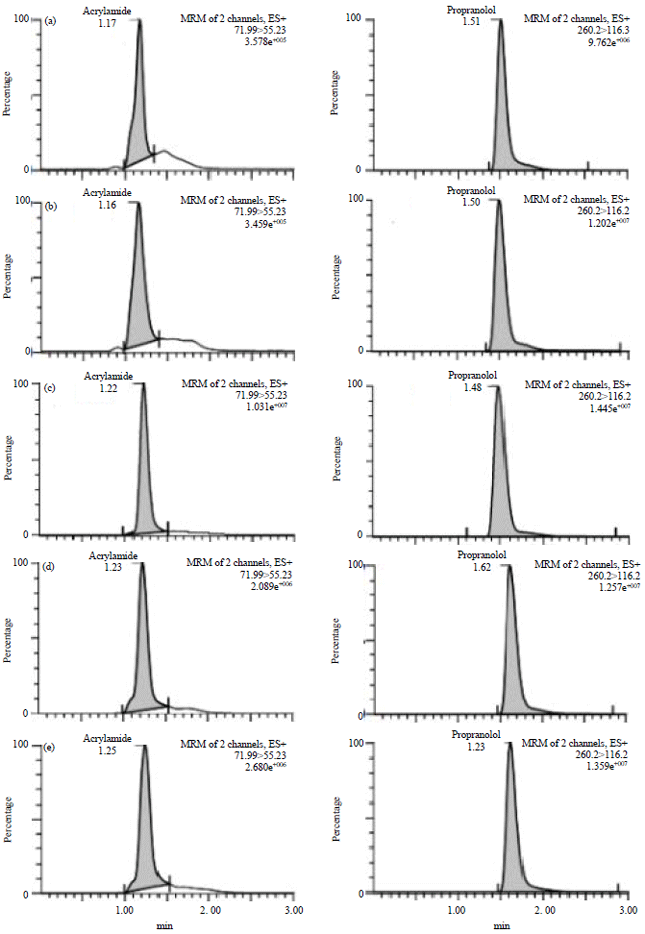
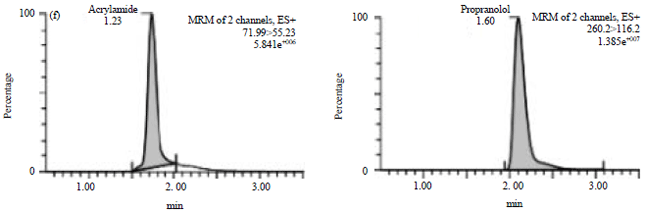
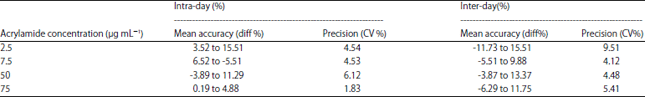
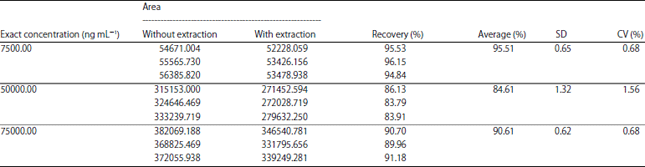
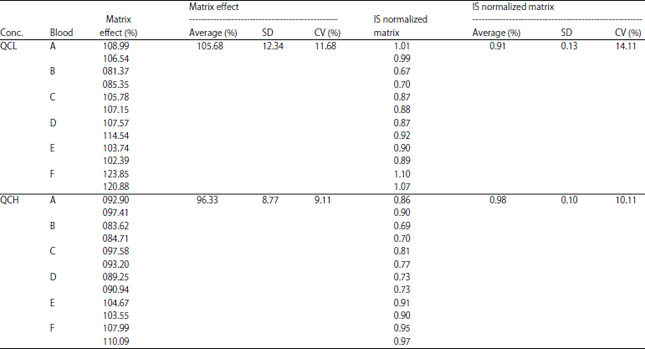
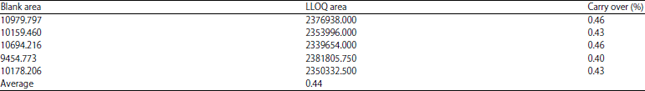
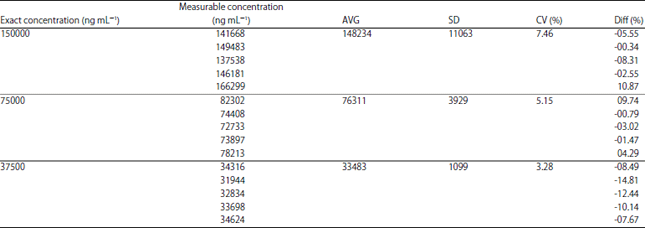
Research Article
Method Validation of Acrylamide in Dried Blood Spot by Liquid Chromatography-tandem Mass Spectrometry
Faculty of Pharmacy, Universitas Indonesia, Depok, West Java, Indonesia
Yahdiana Harahap
Faculty of Pharmacy, Universitas Indonesia, Depok, West Java, Indonesia
LiveDNA: 62.20262
Eme S. Sitepu
Faculty of Pharmacy, Universitas Indonesia, Depok, West Java, Indonesia