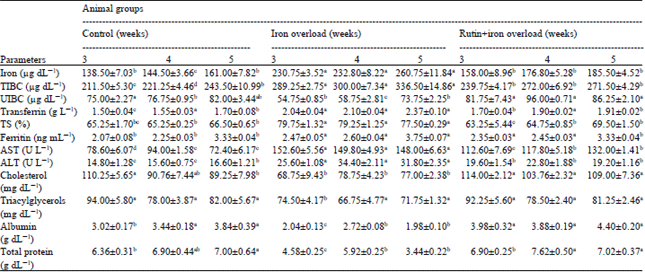
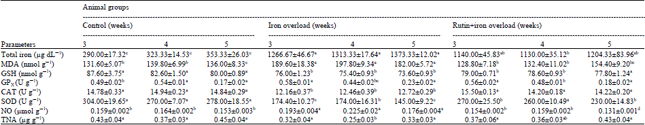
Research Article
Ameliorating Role of Rutin on Oxidative Stress Induced by Iron Overload in Hepatic Tissue of Rats
Department of Biochemistry, Faculty of Veterinary Medicine, Benha University, Moshtohor, Egypt
Mohammed El-said Azab
Department of Physiology, Faculty of Veterinary Medicine, Benha University, Moshtohor, Egypt
Soheir Kamal El-Shall
Department of Biochemistry, Faculty of Veterinary Medicine, Benha University, Moshtohor, Egypt