Research Article
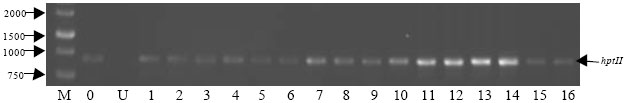
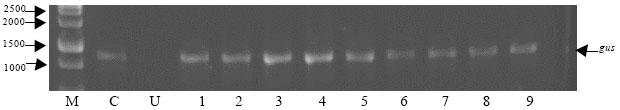
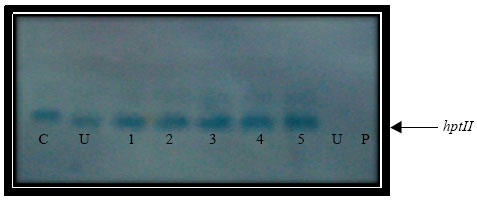
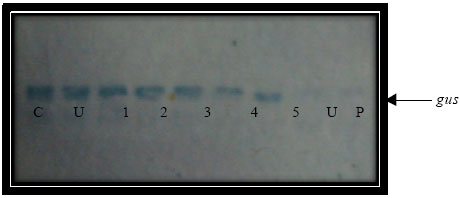
Plant Genetic Transformation Efficiency of Selected Malaysian Rice Based on Selectable Marker Gene (hptII)
Institute of BioScience, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
Ho Chai Ling
Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular, Science Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
Faridah Qamaruz Zaman
Department of Biology, Faculty of Science, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
Mahmood Maziah
Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400, UPM Serdang Selangor, Malaysia