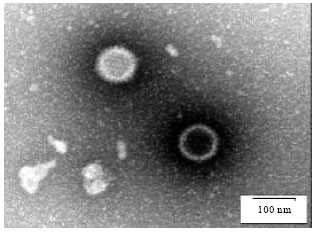
Research Article
Isolation and Characterization of BoHV-1 from Seropositive Cows after Inducing Artificial Stress in West Bengal, India
Department of Veterinary Epidemiology and Preventive Medicine, West Bengal University of Animal and Fishery Sciences, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India
Chanchal Guha
Department of Veterinary Epidemiology and Preventive Medicine, West Bengal University of Animal and Fishery Sciences, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India
Dhruba Chakraborty
Institute of Animal Health and Veterinary Biologicals, Government of West Bengal, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India.
Biplab Pal
Institute of Animal Health and Veterinary Biologicals, Government of West Bengal, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India.
Ujjwal Biswas
Department of Veterinary Epidemiology and Preventive Medicine, West Bengal University of Animal and Fishery Sciences, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India
Amaresh Chatterjee
Department of Veterinary Epidemiology and Preventive Medicine, West Bengal University of Animal and Fishery Sciences, 68 K.B. Sarani, Kolkata-700 037, West Bengal, India
Patricia Koenig
Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, S�dufer10, 17493 Greifswald-InselRiems, Germany.
Martin Beer
Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, S�dufer10, 17493 Greifswald-InselRiems, Germany.