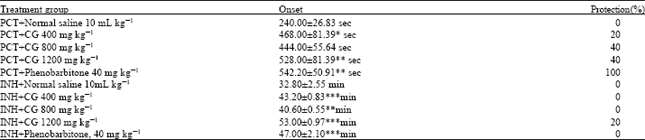
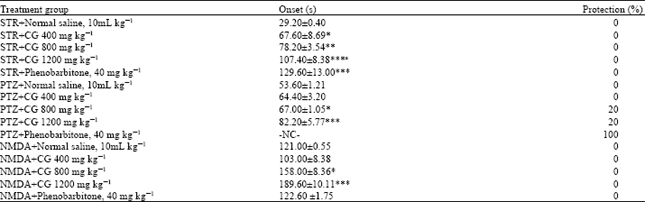
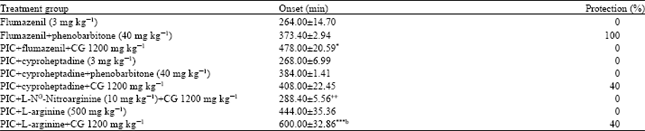
Research Article
Anticonvulsant, Anxiolytic and Hypnotic effects of Aqueous Bulb Extract of Crinum glaucum A. Chev (Amaryllidaceae): Role of GABAergic and Nitrergic Systems
Department of Pharmacology, Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, PMB 12003, Idi-Araba, Lagos, Nigeria
Sunday O. Olayemi
Department of Pharmacology, Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, PMB 12003, Idi-Araba, Lagos, Nigeria
Abidemi R. Idowu
Department of Pharmacology, Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, PMB 12003, Idi-Araba, Lagos, Nigeria