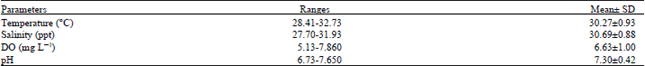
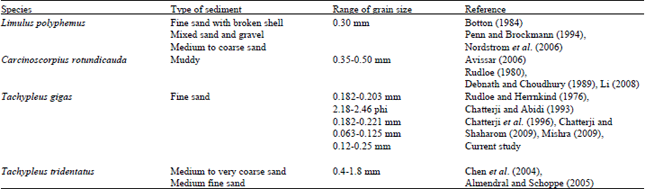
Research Article
Horseshoe Crab, Tachypleus gigas (Müller, 1785) Spawning Population at Balok Beach, Kuantan, Pahang, Malaysia
Laboratory of Marine Science and Aquaculture, Institute of Bioscience, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
A. Christianus
Laboratory of Marine Science and Aquaculture, Institute of Bioscience, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
S. Shakibazadeh
Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
P. Hajeb
Center of Excellence for Food Safety Research, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia