Research Article
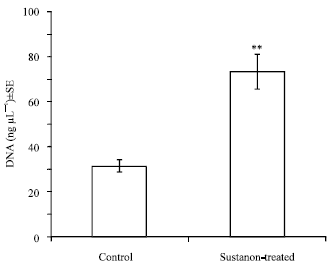
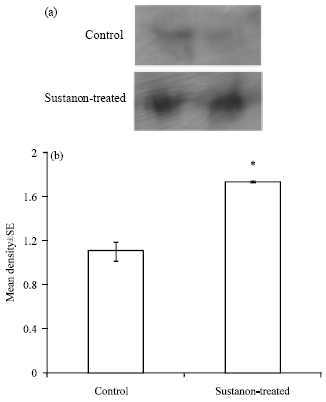
Effects of Sustanon on the Distribution of Satellite Cells and The Morphology of Skeletal Muscle Fibers During Maturation
Department of Anatomy, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Mohammed H. Aldirawi
Department of Anatomy, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan









cheap steroids uk Reply
I bought testosterone boosters to help my muscles, increase my energy, and metabolism how do i block estrogen?