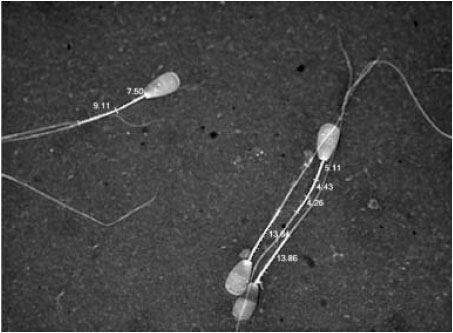
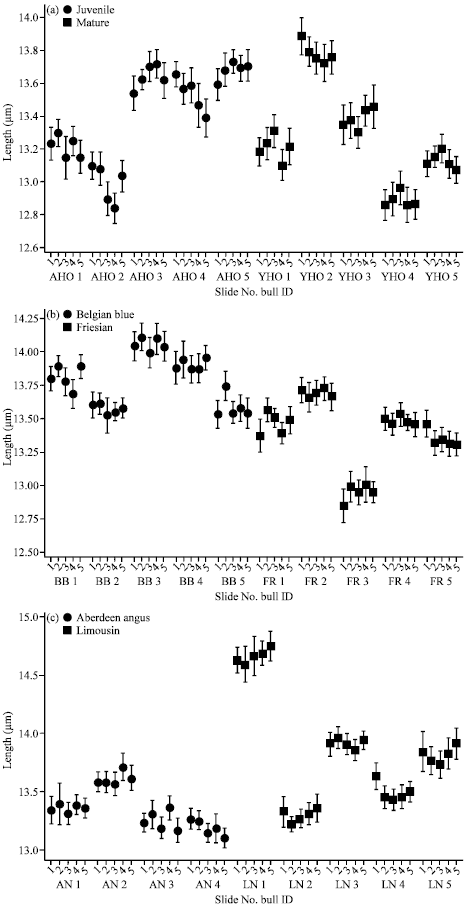
Research Article
Mid-piece Length of Spermatozoa in Different Cattle Breeds and its Relationship to Fertility
University of Liverpool School of Veterinary Science, Leahurst Campus, Chester High Road, Neston. CH64 7TE, UK
S. G. Revell
Genus Breeding Limited, Freezing Unit, Llanrhydd, Ruthin, UK
C. G. Argo
University of Liverpool School of Veterinary Science, Leahurst Campus, Chester High Road, Neston. CH64 7TE, UK
R. D. Murray
University of Liverpool School of Veterinary Science, Leahurst Campus, Chester High Road, Neston. CH64 7TE, UK