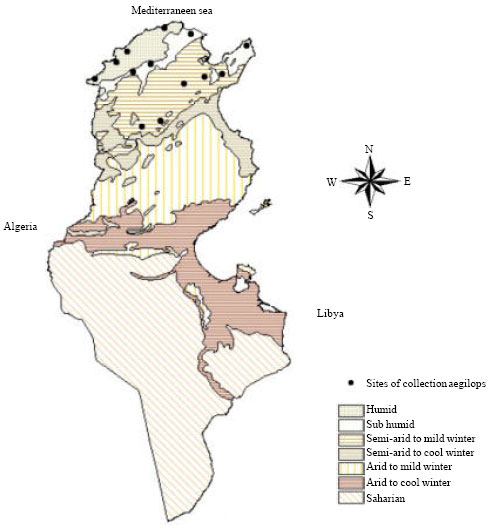
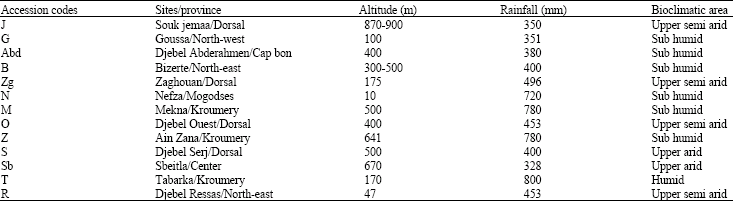
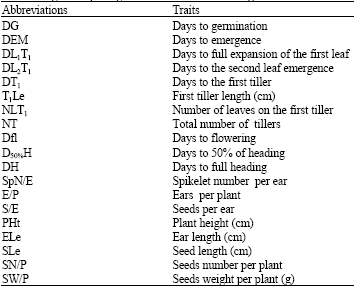
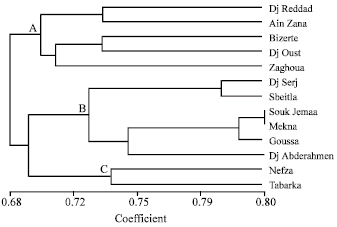
Research Article
Evaluation of Genetic Diversity in Aegilops geniculata Roth Accessions using Morphological and RAPD Markers
Laboratory of Botanic of Inrat, Hedi Karray Street, 2049 Ariana, Tunisia
Mohamed Salah El Gharbi
Laboratory of Cereals Genetic of Inrat, Hedi Karray Street, 2049 Ariana, Tunisia
K. Mguis
Laboratory of Botanic of Inrat, Hedi Karray Street, 2049 Ariana, Tunisia
Mohamed El Gazzah
Genetic and Bio-Resources Unit, University of Sciences of Tunis, Tunis, Tunisia
N.B. Brahim
Laboratory of Botanic of Inrat, Hedi Karray Street, 2049 Ariana, Tunisia