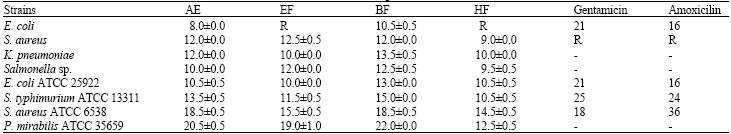
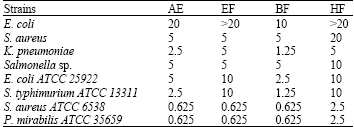
Research Article
Antioxidant and Antibacterial Activities of Combretum nioroense Aubrév. Ex Keay (Combretaceae)
Laboratoire de Biochimie et Chimie Appliquées, UFR/SVT, Université de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso
H. Millogo-Kone
Institut de Recherche en Sciences de la Sante, Centre National de la Recherche Scientifique et Technologique, 03 B.P. 7192, Ouagadougou 03, Burkina Faso
A. Lamien-Meda
Institut for Applied Botany and Pharmacognosy, University of Veterinary Medicine Vienna, Veterinärplatz 1, A-1210 Vienna, Austria
C.E. Lamien
Animal Production Unit, FAO/IAEA Agriculture and Biotechnology Laboratory, IAEA, Vienna, Austria
M. Lompo
Institut de Recherche en Sciences de la Sante, Centre National de la Recherche Scientifique et Technologique, 03 B.P. 7192, Ouagadougou 03, Burkina Faso
M. Kiendrebeogo
Laboratoire de Biochimie et Chimie Appliquees, UFR/SVT, Universite de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso
S. Bakasso
Laboratoire de Biochimie et Chimie Appliquees, UFR/SVT, Universite de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso
M. Yougbare-Ziebrou
Laboratoire de Biologie et d’Ecologie Vegetales, UFR/SVT, Universite de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso
J. Millogo-Rasolodimby
Laboratoire de Biochimie et Chimie Appliquees, UFR/SVT, Universite de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso
O.G. Nacoulma
Laboratoire de Biochimie et Chimie Appliquees, UFR/SVT, Universite de Ouagadougou, 09 BP 848 Ouagadougou 09, Burkina Faso