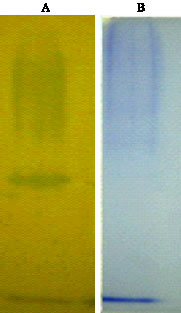
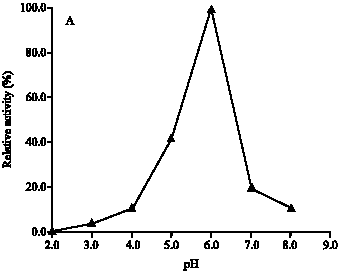
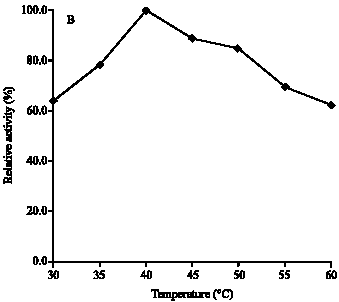
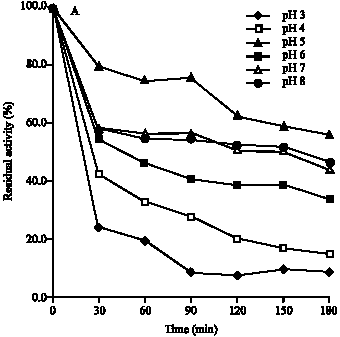
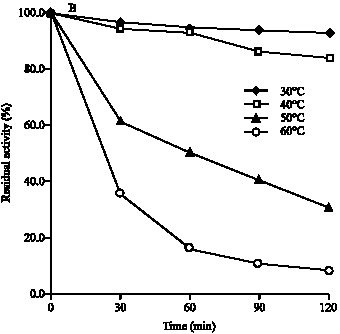
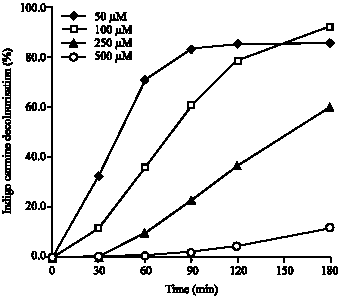
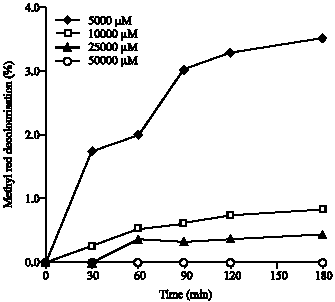
Research Article
Evaluation of Dyes Decolourisation by the Crude Enzyme from Pleurotus sajor-caju Grown on Sorghum Seed Media
Protein and Enzyme Technology Research Unit, Department of Chemistry, Faculty of Science, Mahasarakham University, Maha Sarakham 44150, Thailand
S. Khammuang
Protein and Enzyme Technology Research Unit, Department of Chemistry, Faculty of Science, Mahasarakham University, Maha Sarakham 44150, Thailand