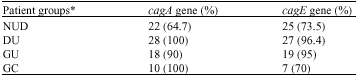Research Article
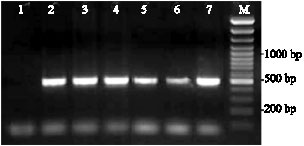
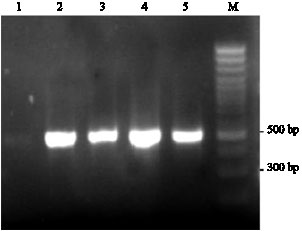
The Prevalence of CagA and CagE Genes in Helicobacter pylori Strains Isolated from Different Patient Groups by Polymerase Chain Reaction
Department of Pathobiology, School of Public Health, Medical Sciences/University of Tehran, Tehran, Iran
M.H. Shirazi
Department of Pathobiology, School of Public Health, Medical Sciences/University of Tehran, Tehran, Iran
R. Ranjbar
Molecular Biology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
M.R. Khorramizadeh
Department of Biotechnology, School of Public Health, Medical Sciences/University of Tehran, Tehran, Iran
N.E. Daryani
Faculty of Medicine, Medical Sciences/University of Tehran, Tehran, Iran
M. Hosseini
Department of Biostatistics, School of Public Health, Medical Sciences/University of Tehran, Tehran, Iran