Clinical Report
Chemotaxonomic Studies on Aegilops L. (Poaceae) In Iran
Department of Botany, Faculty of Sciences, University of Shahrekord, Shahrekord, Iran
INTRODUCTION
Aegilops L. is one of the genuses of Poaceae and Triticeae having 13 species in Iran (Bor, 1968, 1970). This genus has an annual life with mainly out crossing breeding system (Waines and Barnhart, 1992). Aegilops has three ploidy levels including diploid (2n = 2x = 14), tetraploid (2n = 4x = 28) and hexaploid (2n = 6x = 42) with x = 7 (Bor, 1968). Due to the fact that Aegilops is a pastoral plant and also some of the Aegilops species are considered as the progenitors for polyploidy Triticum L. species and cause the evolution of Triticum genus, it is used as a source of resistance, salt and drought tolerance in wheat breeding (Baum and Apples, 1992).
The complexes occurred among the species were due to the high similarity and the hybridization between the Aegilops species (Lange and Jochemsen, 1992a), these evidences have obscured the morphological limits of the species and caused taxonomic confusions in the number of species in the genus Aegilops (Morrison, 1993a, b). High levels of variations have been observed in this genus, as well (Waines and Barnhart, 1992). Therefore the genus Aegilops has been of considerable interest among plant taxonomists (Keshavarzi and Rahiminejad, 2003).
Chemo taxonomically, in Triticeae several studies have been done on seed proteins (Waines and Johnson, 1971; Baum, 1977; Esen and Hilu, 1991; Morel, 1994; Chen et al., 1997). Controversially, in Aegilops genus a few studies were done on prolamin (Masci et al., 1992; Waines and Barnhart, 1992). Seed proteins are good markers for assessing taxonomic and phylogenetic relationships at various levels of species through subfamilies (Masci et al., 1992; Chen et al., 1997). Prolamin marker occurring in seed of cereals was applied in Tribe and Family and solved the complexity of taxonomy and grass phylogeny (Waines and Barnhart, 1992). Oda (1994) reported that friabilin is one of the seed proteins in Aegilops, which can be employed in detecting taxonomic position of the genus and species. Among the polyploidy species, the width of each protein bands is controlled by specific genome (Masci et al., 1992). In addition, Holubec and Dvoracek (2005) emphasized the variability among Aegilops tauschii Coss. and Ae. triuncialis L., showing the relationships in different ploidy levels and their variability. Another finding is concerned with populations of Ae. cylindrica Host. in Hungary, where they have showed a high genetic diversity in gliadin patterns (Vorosvary et al., 2000).
Due to the wide range of variation among the Aegilops species and insufficient information about the prolamin in the genus, it seems necessary to be evaluated taxa of the genus Aegilops. Regarding incomplete prolamin pattern studies among Aegilops species in Iran, the necessity for biosystematic investigations seems to be very important in this country. So the purposes of this study are: 1) identifying the variation of prolamin patterns among Aegilops species, 2) Displaying the taxonomic position via prolamin among Aegilops species and 3) Determining the relationships between tetraploid and diploid species in Iran.
MATERIALS AND METHODS
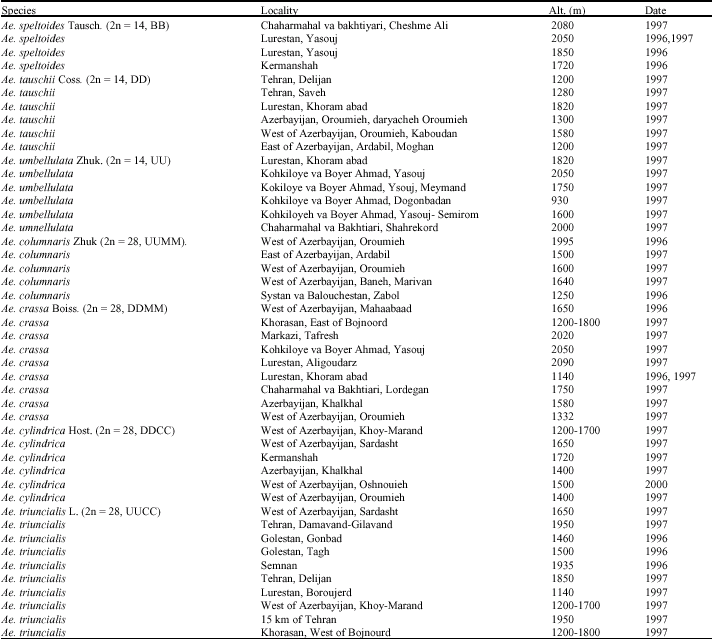
The Aegilops species were collected (seven species, 46 accessions) from natural habitats of Iran in July and August 1995 and 1996 (Fig. 1, Table 1). All the accessions were grown in the research field at Isfahan University in October 1996. The seed prolamin of each accession of Aegilops species was extracted by 60% isopropanol, 5% 2-mercaptoethanol and 10% Sodium Dodecyle Sulphate (SDS) (Esen and Hilu, 1991; Shewry, 1993; Morel, 1994; Wang et al., 1994). Then the SDS-PAGE (12%) and Molecular Weight Marker 10 μL (11-B-064-03A) were applied. In order to evaluate the taxonomic position of Aegilops species, each prolamin bands of seven species were studied among the
 | |
| Fig. 1: | Distribution of Aegilops species in natural habitat of Iran |
| Table 1: | Locations of the Aegilops species in natural habitats of Iran |
 | |
accessions, using Rf. value (the migration distance of the band/distance of solvent front) and Molecular Weight (MW) of prolamin bands. The MW of the protein band in each accession has been calculated by diagram of MW of marker (kDa)/migration distance of marker (cm) (Apaydin and Bilgener, 2000; Sharifnia and Assadi, 2003; Gulen and Eris, 2004). Four SDS-PAGE among different accessions of each species were performed (Fig. 2a, b). The statistical procedures such as Cluster analysis (between grouped linkage method and squared Euclidean Distance) and descriptive analysis were run using the SPSS V.14.
RESULTS
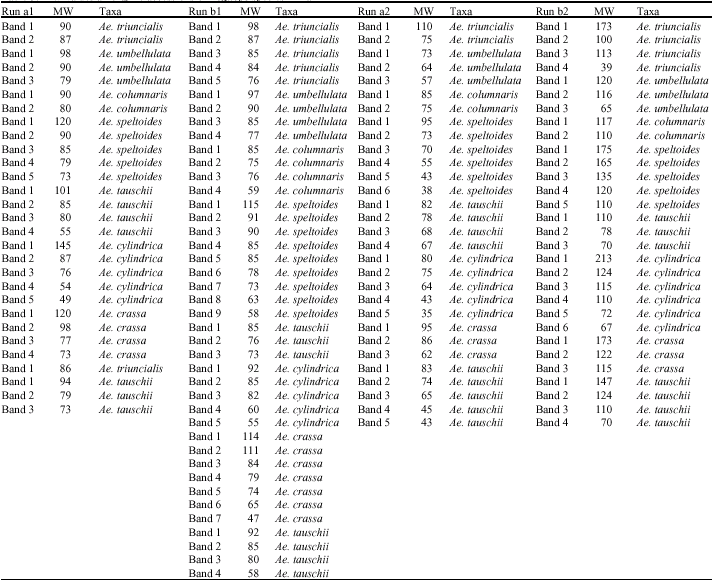
The electrogram of prolamin patterns showed 29, 41 and 30 bands which were observed among the accessions of Aegilops species (Fig. 2a-d). According to Rf. values, the highest amount of Rf.1 (run a1) is 1.4 (Ae. cylindrica) and 1.3 (Ae. tauschii), the lowest Rf.1 is 0.33 and is observed in Ae. cylindrica (Fig. 2a, Table 2). The highest Rf.2 (run b1) is 1.4 (Ae. cylindrica) (Fig. 2b, Table 2). The lowest Rf.2 is 0.52 and was observed in Ae. speltoides (Fig. 2b, Table 2). The highest Rf.3 (run a2)
is 1.64 (Ae. cylindrica), 1.58 (Ae. tauschii) and 1.54 (Ae. speltoides) (Fig. 2c, Table 2). The lowest Rf.3 is 0.64 which was found in Ae. speltoides (Fig. 2c, Table 2). The highest Rf.4 (run b2) is 2.22 (Ae. triuncialis), 1.28 (Ae. umbellulata), 1.25 (Ae. cylindrica) (Fig. 2d, Table 2). The lowest Rf.4 was observed in Ae. cylindrica (0.17) and Ae. speltoides (0.22) (Fig. 2d, Table 2).
In Fig. 2a the lowest M.W is 49 kDa and the highest M.W is 145 KDa that are related to Ae. cylindrica. In Fig. 2c, the highest MW is related to Ae. triuncialis (110 kDa). The lowest MW is related to Ae. cylindrica (35 kDa). In Fig. 2b, the highest MW is in Ae. speltoides (115 kDa) and Ae. crassa (114 kDa). The lowest MW is related to Ae. crassa (47 kDa). Most of the weight in this electrogram has Low MW. As Fig. 2d displays, the highest MW can be found in Ae. cylindrica (213 kDa) and Ae. speltoides (175 and 165 kDa). The lowest MW can be found in Ae. triuncialis (39 kDa) (Table 3).
The results of cluster analysis show that there were two groups comprised based on Rf. data (Fig. 3b); 1) Ae. triuncialis, Ae. columnaris, Ae. umbellulata Ae. crassa and Ae. tauschii, 2) Ae. tauschii, Ae. speltoides and Ae. cylindrica. Noticeably, two accessions of Ae. tauschii were separated from each other (Fig. 3b).
 | |
| Fig. 2a-d: | Diagram of prolamin bands among the accessions of Aegilops species. a: first run, b: second run, c: third run, d: fourth run. ta: tauschii, um: umbellulata, co: columnaris, sp: speltoides, cy: cylindrica, tr: triuncialis, cr: crassa |
| Table 2: | Rf. values among the accessions of Aegilops species in Iran |
 | |
 | |
| Fig. 3a-b: | Dendrogram (cluster analysis) among the Aegilops species using MW (a) and Rf. (b) Values. ta: tauschii, um: umbellulata, co: columnaris, sp: speltoides, cy: cylindrica, tr: triuncialis, cr: crassa |
Based on MW marker (Table 3), the results of clustering showed that two groups were comprised 1) Ae. umbellulata, Ae. crassa, Ae. tauschii and Ae. tauschii, Ae. triuncialis, Ae. columnaris, 2) Ae. speltoides, Ae. cylindrica (Fig. 3a).
In Table 4 and 5, the highest C.V was observed in Ae. crassa and Ae. triuncialis and the lowest was in Ae. triuncialis.
DISCUSSION
Based on the results of this study, among the diploid and tetraploid Aegilops species, there would be observed a similar origin (Fig. 2a-d). The sub-units of prolamin depict two regions based on the fast and slow movement which is observed among the accessions of Aegilops species. In this study, all of the electromorphic patterns of prolamin have LMW from 35-76 kDa (Table 3). Similarly, Zhang et al. (2006) observed that in the wild Aegilops species high molecular mass storage proteins` (glutenin) sub-unit possess a mobility which is slow and fast. In comparison to the other Aegilops
| Table 3: | MW values among the accessions of Aegilops species in Iran |
 | |
species, Ae. cylindrica and Ae. speltoides accessions have the highest MW of prolamin which are 213-145 kDa, 175- 135 kDa, respectively (Table 3). Wan et al. (2002), using SDS-PAGE of total protein fractions from single seed of Ae. cylindrica, claimed the presence of three bands corresponding to HMW sub-units of glutenin. In accessions of Ae. cylindrica similar patterns of HMW sub-units with similar mobility were observed, which is in accordance with our results. In addition, the available studies have indicated that Aegilops is a rich source for novel variant of HMW seed protein sub-units. Lagudah and Halloran (1988) reported that all gliadin sub-units from Ae. umbellulata have HMW. The results of this study indicate that Ae. umbellulata have HMW prolamin sub-units (Table 3).
The variability among the diploid and polylploid levels showed that in estimating the CV among the accessions of Ae. tauchii and Ae. triuncialis, low CV is observed (Table 4, 5). Buren (2001) reported that the
| Table 4: | Descriptive analysis among the accessions of Aegilops species using Rf. |
 | |
| A: Standard Deviation, B: Coefficient of Variation | |
| Table 5: | Descriptive analysis among the accessions of Aegilops species using MW |
 | |
| A : Standard Deviation, B: Coefficient of Variation | |
alleles of seed storage proteins` (gliadin) genes are clustered in accessions of Ae. tauschii in Caspian region. Additionally, among the subspecies accessions of Ae. taucchii from Iran, these genes were of high similarity, which is due to the high gene flow present among them (Buren, 2001), thus classified together. Moreover, according to Kharazian (2007) Ae. tauschii (diploid species) have the lowest variation in different regions of Iran. Also, according to Keshavarzi and Rahiminejad (2003), some of the diploid species seemed to have a pure genome due to high gene flow resulting from the lower variation among the diploid species. Moreover, in some of the accessions of Ae. triuncialis and Ae. crassa, the CVs are higher than those of other species. The evidences demonstrate that some of the tetraploid species are more adapted and variable than the diploid species, confirming the evaluations of Kaminski et al. (1990), Salomon and Lu (1992) and Van Slageren (1994). Also Holubec and Dvoracek (2005) reported that tetraploid species have special bands which cause variability among them. Based on ploidy levels, Holubec and Dvoracek`s (2005) research indicates that in tetraploid species, Ae. triuncialis have the lowest MW Among the tetraploid species of this study, some of the accessions of Ae. triuncialis and Ae. crassa have the lowest and highest MW In diploid species, Ae. speltoides has the highest MW, but Ae. tauschii has the lowest MW (Table 2, 3).
The cluster analysis indicated that in most of the data analyses such as Rf. values and MW, Ae. triuncialis and Ae. tauschii were grouped together (Fig. 3a-b), Yue et al. (2005) reported that between Ae. tauschii and Ae. triuncialis LMW-Td2 (glutenin subunit gene) is a putative pseudo gene as it is proved to be extremely homologous due to the similarity between them, so it seems to be due to the relationships of prolamin bands observed in these two species. Wan et al. (2002) also reported that Ae. tauschii (diploid) is one of the parents of Ae. cylindrica (tetraploid) which have characteristic two-bands HMW-glutenin pattern, particularly, Ae. cylindrica has three band patterns. In this study, in the application of Rf. data, Ae. tauschii (DD) and Ae. cylindrica (DDCC) have similar genome which were grouped together (Jaaska, 1981, 1993) but in the MW are separated. These two species have HMW and LMW of prolamin (Table 2, 3). Noticeably, in this study prolamin patterns were similar among the Ae. tauschii (DD, 2n = 2x = 14) and Ae. crassa (DDMM, 2n = 4x = 28), in most of the cluster analyses, these two species were grouped together (Fig. 3a-b). Eig (1929), Masci et al. (1992), Lange and Jochemsen (1992a, b) and Van Slageren (1994) showed that Ae. tauschii is similar to Ae. crassa. In addition, some of the species can not be separate morphologically, in cluster analyses they can be grouped together but have exactly been distinguished, such as Ae. columnaris (UUMM, 2n = 4x = 28) and Ae. umbellulta (UU, 2n = 2x = 14) which in previous morphological studies, Eig (1929), Bor (1968, 1970) and Van Slageren (1994) considered them as similar species. Existing high genomic similarity between Ae. columnaris (UUMM) and Ae. triuncialis (UUCC, 2n = 4x = 28), they are grouped together which is in accordance with the results of Eig (1929) and Van Slageren (1994).
The relationships among two ploidy levels such as tetraploid and diploid were observed, so Ae. triuncialis (UUCC, 2n = 4x = 28), as a tetraploid species in the mainly procedure of data analyses, was grouped with Ae. tauschii (DD, 2n = 2x = 14) and Ae. umbellulata (UU, 2n = 2x = 14) (Fig. 3). Yue et al. (2005) and Holubec and Dvoracek (2005) showed that the sub-units of glutenin are extremely homologous in Ae. tauschii and Ae. triuncialis. In addition, Ae. triuncialis is a natural allopolyploid of Ae. caudata L. and Ae. umbellulata, but two new slow moving bands are present in this allopolyploid species, which are not observed in parents (Waines and Johnson, 1971). These bands may be caused by the act of tetraploidity. Using genome analysis and anatomical studies, Lange and Jochemsen (1992a, b) and Kharazian (2007) proved that Ae. triuncialis was related to Ae. umbellulata and some of the accessions of Ae. tauschii were fewer related to Ae. speltoides and these evidences have been observed in the results of the Cluster analysis in this study (Fig. 3a-b). Also, even the genomic formulae of Ae. tauschii (DD, 2n = 2x = 14) and Ae. crassa (DDMM, 2n = 4x = 28) and Ae. triuncialis (UUCC, 2n = 4x = 28) and Ae. umbellulata (UU, 2n = 2x = 14) show the similarity between them (Fig. 3a-b) and support a close relationship between the two species (Eig, 1929; Van Slageren, 1994; Keshavarzi and Rahiminejad, 2003; Kharazian, 2007). In addition, the Cluster analysis shows that some of the diploid and tetraploid species have high gene flow, which is referred to as their `taxonomic complexes`. Also, despite similar genomes among the tetraploid species such as Ae. cylindrica (DDCC) and Ae. crassa (DDMM, 2n = 4x = 28), these are separated. Ae. columnaris (UUMM, 2n = 4x = 28) and Ae. crassa (DDMM) have similar genome, in most of the clusters they are grouped together (Fig. 3a-b). According to Kharazian (2007), Ae. triuncialis (tetraploid species), Ae. crassa (tetraploid species) and Ae. cylindrica (tetraploid species) are widely distributed regarding the highest variation. These evidences show that Aegilops genomes have high variability, which is an excellent gene pool to plant breeding.
CONCLUSION
Finally, regarding the results of this study, Aegilops species are becoming increasingly important as a potential source of valuable traits that can be used in wheat (T. aestivum) breeding (Bultynck et al., 2003). Also, prolamin patterns can be mentioned as an appropriate marker to display the chemotaxonomic position and the genomic relationships among them.