Research Article
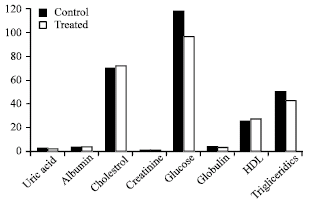
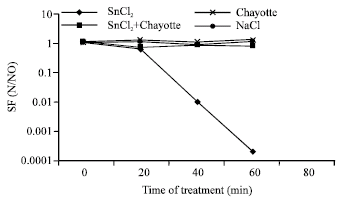
Effects of a Chayotte (Sechium edule) Extract (Macerated) on the Biochemistry of Blood of Wistar Rats and on the Action Against the Stannous Chloride Effect
Universidade do Estado do Rio de Janeiro, Instituto de Biologia Roberto Alcantara Gomes, Departamento de Biofísica e Biometria. Av. 28 de setembro, 87, Rio de Janeiro, RJ 20551-030, Brazil
M.C.L. Almeida
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
M.F.M.C. Coura
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
S.D.D. Vasconcelos
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
P.R.A. Siqueira
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
R.M. Duarte
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
J.S. Rodrigues
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
J.C.S. Oliveira
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
M.L. Fernandes
Universidade Estácio de Sá. Centro de Ciências da Saúde, Campus R9/Taquara, Laboratórios de Semiologia e Semiotécnica e Fisiologia do Exercício, Rua André Rocha, 838, Rio de Janeiro, RJ 22710560, Brazil
M. Bernardo-Filho
Universidade do Estado do Rio de Janeiro, Instituto de Biologia Roberto Alcantara Gomes, Departamento de Biofísica e Biometria. Av. 28 de setembro, 87, Rio de Janeiro, RJ 20551-030, Brazil