Research Article
Utilization of Dimethoate by Wild Type Pseudomonas putida from Polluted Sites in Iran
Department of Clinical Biochemistry, Faculty of Medicine, Medical Science University, Zanjan, Iran
Organophosphorus compounds (OPs) are the widely used pesticides, suggesting for about 34% of world-wide insecticide sales (Zhang and Qiao, 2002). Different reports have recorded that a wide range of natural ecosystems may be polluted. These chemicals possess high toxicity and must remove. Bioremediation offer an efficient technique for detoxification of polluted ecosystems. Those high toxic residues can be simply cleansed in environment (Singh and Walker, 2006; Zhang et al., 2005). Dimethoate one of OPs is widely applied in agriculture. It is highly toxic (Al-Jughbir et al., 1992) is possibly teratogenic and may promote cancer (Racke and Coats, 1988). Some resistant bacteria, capable of degrading dimethoate which have been isolated and characterized (Deb-Mandal et al., 2005). Microbial degradation of dimethoate (Deb-Mandal et al., 2003) and dimethoate resistant bacteria in aquatic bodies have been reported (Deshpande et al., 2001; Deb-Mandal et al., 2002). The resistant bacteria had been isolated, which harbored OP degrading plasmids (Nazarian and Mousawi, 2005). We were characterized the utilization of dimethoate as phosphorus and energy requirement by wild type and gram negative strains. Dimethoate utilization was confirmed indirectly with anti-choline esterase activity (Snyder and Walker, 1999). The strains had been selected, among OP resistant bacteria in the field of which had been polluted with OPs.
Enrichment medium: Two resistant bacteria P. putida (P) and Flavobacterium sp. (F) were incubated in 16 (50 mL) flasks with sterile mineral salt solution (MS) containing Tris [hydroxymethyl amino methane (50 mM)], KCl (207 mM), MgSO4 (0.8 mM), NH4Cl (40 mM), FeCl3 (0.005 mM), DH2O (1L), PH = 7.2-7.4 (duplicated). MS was enriched with glucose (0.3 g L-1) and organophosphates: Guthion (0.1 g L-1), dimethoate (0.2 g L-1) in two forms (hydrolyzed or intact) and also 2 (50 mL) flasks were used without glucose (control). All flasks were aerobically shaked (200 rpm) at 30deg;C for 48 h. The bacterial growth was determined as turbidity measurement of optical density by spectrophotometer at 500 nm. The cultures were plated for colony forming with 105 cells on semisolid MS plates containing OP. Also the minimum inhibitory concentrations of dimethoate was determined in nutrient broth at concentration of 10 folds more than in MS.
Utilization OP pesticides: Four resistant strains (105 cell) as single and mixed culture were separately incubated into sterile 75 mL MS containing 0 and 2 g L-1 dimethoate and 2 g L-1 Orthophosphate (inorganic phosphate) in duplicate cotton plugged 100 mL flasks, were continuously shaken (200 rpm) for 48 h at 30deg;C and monitored for turbidity and colony counts every 24 h, also total and inorganic phosphorus (Burtis and Ashwood, 2006), extra cellular and intra cellular proteins were determined after centrifugation (MSE 25, Beckman, fixed angle rotor, 20000 g, 30 min, 4deg;C) and ultrasonication of cultures (MSE-150 W, power: 70%, microprobe with range of 22.5 micron). The reagents were used for phosphorus determination: Ascorbate (10%), NH4MoO4, 4H2O (42% in 1N H2SO4) were mixed in ratio of 1: 6 with Mg [(NO)3]2, 2H2O (10% in 95% C2H5OH), HCL (4%). The glass wares were completely treated overnight by nitric acid (5N) for cleaning of any phosphorus and washed entirely by D H2O and dried by oven.
Bacterial remediation of dimethoate: The four resistant strains (P. putida, P. fluorescence, Flavobacterium sp. and F. breve) were tested for detoxification of dimethoate as single and mixed culture were incubated in MS containing dimethoate (2 g L-1) at 30deg;C for 48 h and the residues assayed for the AChE activity (as Ell man method), by spectrophotometers (Perkin Elmer 35 Junior UV-Vis 554), in comparison with parallel sterile MS incubations of dimethoate (0.0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 and 2.0 g L-1) without bacterial culture.
Plasmid manipulation: The P as resistant strain to dimethoate was shakely treated with acridine orange (0.3 g L-1) in MS at 30deg;C for 48 h. The resistant strains were confirmed by colony forming on MS plates containing dimethoate in comparison with parallel MS control plates without dimethoate. Also the resistant strains had been characterized for antibiotics of which suppose to linked with dimethoate degrading plasmids. The antibiotic resistance was used to differentiate the dimethoate sensitive strains. Dimethoate degrading plasmids were trans-conjugated to sensitive stains and confirmed by replica plating technique.
Enrichment medium: The high OP resistant strains were incubated in MS medium enriched with OPs like dimethoate and guthion in two forms (hydrolyzed and intact). The bacterial growth was observed as turbidity at 500 nm of optical density. The hydrolyzed form of OPs was not showed significant difference with intact form although slightly promoted the bacterial growth. Glucose was needed in guthion case but dimethoate solely sufficient (Fig. 1). The resistant strains P. putida and Flavobacterium sp. were also exhibited the high growth at 2 g L-1 of dimethoate. P. Putida were showed the optimal growth at 1.0 and 2.0 g L-1 of dimethoate in comparison with inorganic phosphate, those confirmed by viable counts of 2x109 and 6x1010 cells mL-1, respectively after 48 h although control experiment (without dimethoate) displayed lower growth activity and colony count of 2x105 cells mL-1, (Fig. 2).
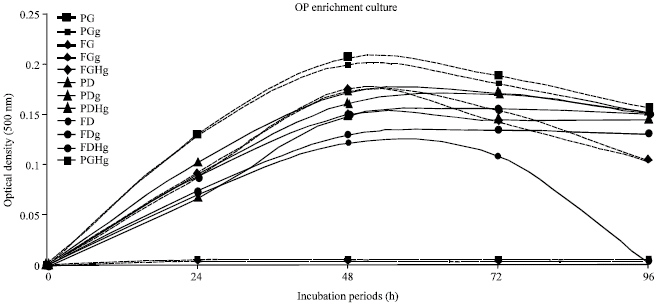 | |
| Fig. 1: | OP Enrichment culture. Two resistant strains [P. putida (P) and Flavobacterium sp. (F)] were incubated in mineral solution (MS) containing Ops: Guthion (0.1 g L-1) and dimethoate (0.2 g L-1), glucose 0.3 g L-1. P and F were optimally exhibited high growth in medium with dimethoate even without glucose, presumably due to dimethoate has used as phosphorus and energy source. Although guthion needs complement as glucose. Two forms of OPs (hydrolyzed and intact have not significant effect. Abbreviations: (P): P. putida, (F): Flavobacterium sp., g: glucose, D: dimethoate, DH: hydrolysed dimethoate, G: guthion, GH: hydrolysed guthion |
| Table 1: | Dimethoate utilization:Dimethoate utilized preferably in comparison with inorganic phosphate as sole phosphorus source, energy and important requirement like protein |
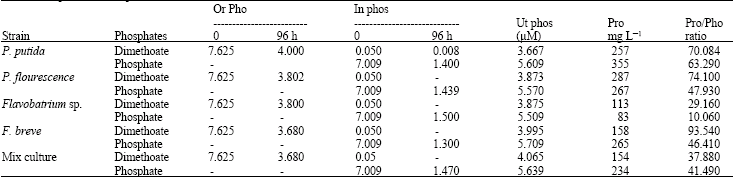 | |
| Notes: Or Phos: Organic phosphorus, In Phos: Inorganic Phosphorus, Ut phos: Utilized Phosphorus, Pro: Synthesized protein | |
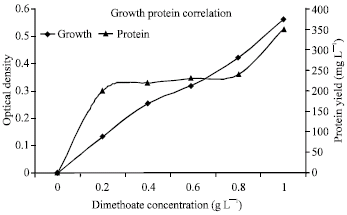 | |
| Fig. 2: | Growth-protein correlation. P. putida was exhibited linear growth turbidity in expose to different concentrations of dimethoate (0.0, 0.2, 0.4, 0.6, 0.8, 1.0 g L-1) and produced proportionally total protein (solid line), but it showed lower growth turbidity (0.286 OD) and total protein (225 mg L-1) in expose to Inorganic phosphate (1.0 g L-1), respectively |
Utilization of OP pesticides: The utilization of dimethoate was exhibited higher growth effects than inorganic phosphates so it directly increased the turbidity (0.560 at 500 nm), colony counts (2x109 cells) and total protein (350 mg L-1) (Fig. 2). P. puttida was utilized dimethoate as choice material in comparison with inorganic phosphates (Table 1). The relation between utilization of phosphates and protein biosynthesis in first two strains was higher than other strain especially in single culture.
Bacterial remediation of dimethoate: The activity of AChE was separately determined in MS containing dimethoate in concentrations of (0.0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.4 and 2.0 g L-1) and also its metabolic products at 2.0 g L-1 after 0, 48 and 96 h of single and mixed culture of P. putida. It was indirectly showed a decrease in dimethoate toxicity 50-100% in 48 and 96 h of bacterial incubation, respectively (Fig. 3).
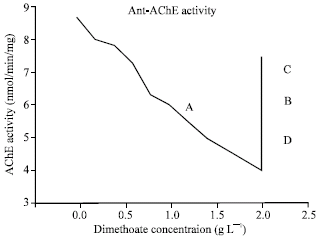 | |
| Fig. 3: | Anti-AChE activity. Dimethoates were inhibited AChE enzyme activity which was showed with different concentrations (0.0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.4 and 2.0 g L-1) (A), in AChE inhibition curve. Because P. putida was utilized dimethoate (2.0 g L-1) in periods 48 and 96 h (B,C) as single or (D) mixed culture which reduced AChE-inhibition 70 and 95%, respectively, with single culture of P. putida |
P. putida and Flavobacterium sp. have been characterized as multi-potent resistant strain for three class of OPs (Nazarian and Mousawi, 2005) also were used to utilization of mentioned OPs. Although phosphotriesterase has used to catalyses the detoxification of phosphotriester pesticides. A short pathway has enabled bacteria to use phosphotriesters as sole source of phosphorus (Foster et al., 2004; Zhang and Qiao, 2002).
The resistance to Ops was due of OP degrading-plasmid and bacterial ability to synthesis catalytic enzymes and utilizing Ops as sole source of phosphorus, energy and presumably a precursor for proteins biosynthesis or other requirement (Digrak and Kazanici, 2001; Raushel, 2002). P. putida F1 can assimilate different aromatic compounds by using related pathways in similar study (Choi et al., 2003). P. putida F1 strains have adapted to assimilate new substrates and the molecular mechanisms of genetic adaptation to an expanded range of aromatic hydrocarbons have determined. The adapted strains which is induced by new growth substrates those are poor inducers of wild-type P. putida F1. These results showed that P. putida is adapted strain capable of growing on substrates (Bertani et al., 2001). The utilization of phosphorus compounds was the basic requirement for energy and biosynthetic pathways like protein. The resistant strains which adapted with dimethoate were promoted at least two folds higher surviving activity than inorganic phosphorus. Because of the principal dimethoate toxicity to AChE activity, the Ops utilization was confirmed by the anti-AChE assay. The linear anti-AChE assay of dimethoate and its reduction after optimal growth of P. putida especially in single culture were confirmed the OP reduction/utilization. In conclusion, the wild type strains with dimethoate degrading plasmids suppose to use as optimal tool of enzyme expressing means that might be apply against anti-nerve agents.
This research was supported by research group of Iranian Ministry of Defense and Institute of Biochemistry-Biophysics in Tehran University. We should acknowledge from related stuff of above centers.