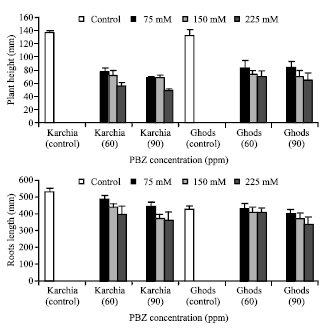
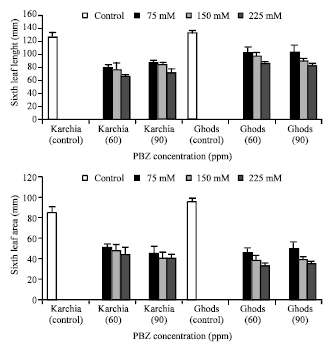
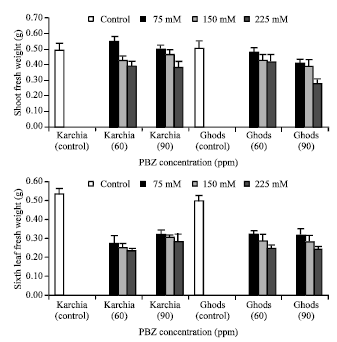
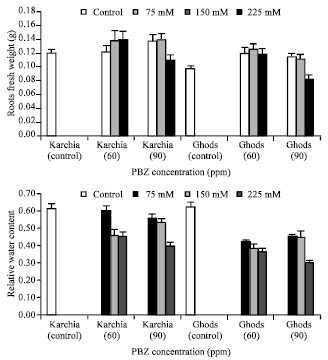
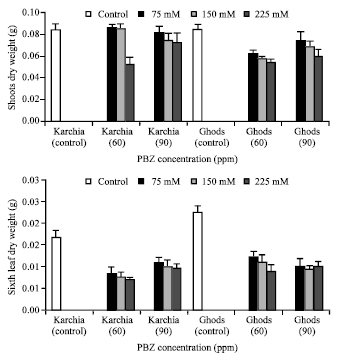
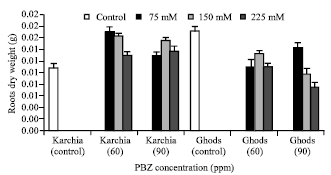
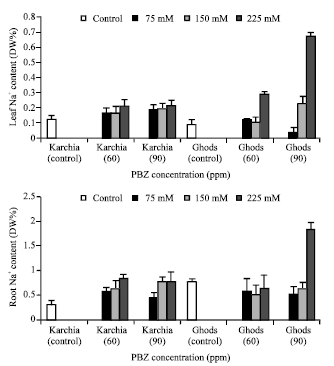
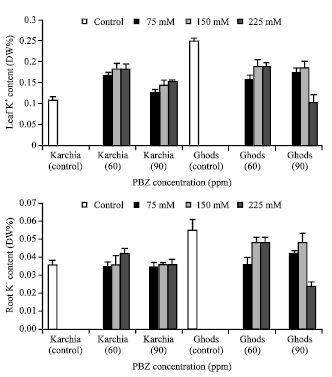
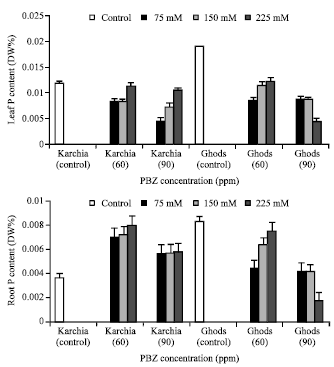
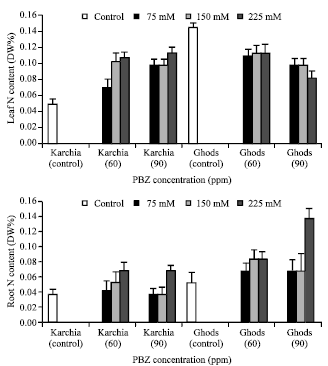
Research Article
Effects of Paclobutrazol and Salt Stress on Growth and Ionic Contents in Two Cultivars of Wheat
Iranian Academic Center for Education, Culture and Research, Alzahra University branch, Tehran, Iran
Khadijeh Kiarostami
Department of Plant Sciences, Alzahra University, Tehran, Iran