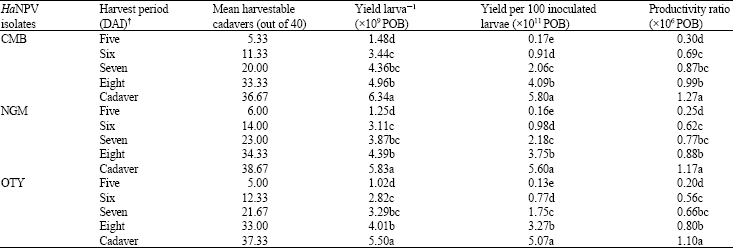
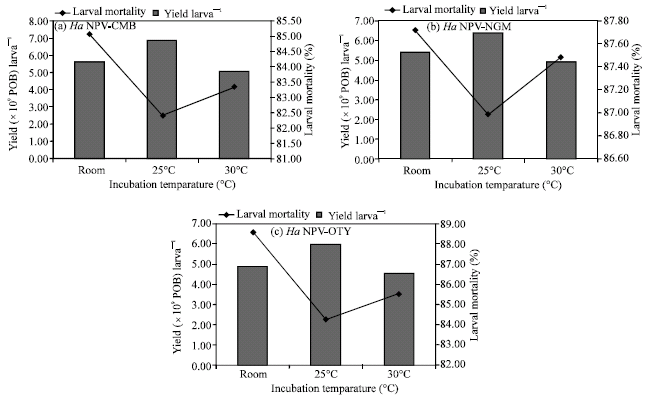
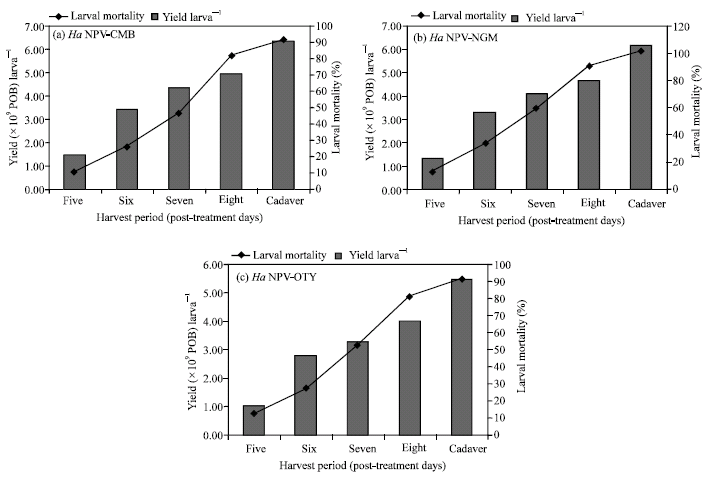
Research Article
Standardization of Mass Production in Three Isolates of Nucleopolyhedrovirus of Helicoverpa armigera (Hübner)
Department of Plant Protection, Maragheh Higher Education Complex, University of Tabriz, Tabriz-5166616471, Iran
R. J. Rabindra
Project Directorate of Biological Control (ICAR), P.B. No. 2491, Bellary Road, Hebbal, Bangalore-560024, India
K. Veenakumari
Project Directorate of Biological Control (ICAR), P.B. No. 2491, Bellary Road, Hebbal, Bangalore-560024, India
G. B. Narabenchi
Pest Control (India) (PCI), Bio-Control Research Laboratories (BCRL), Bangalore-560064, India