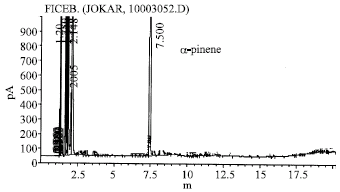
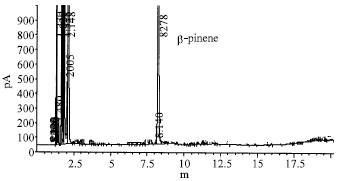
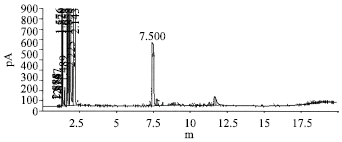
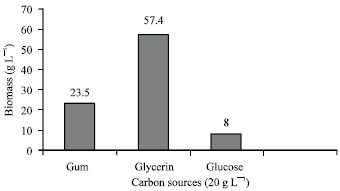
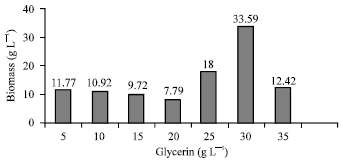
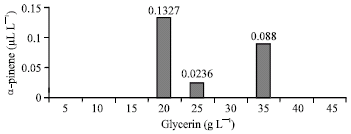
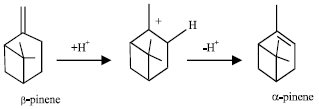
Research Article
Application of Biotransformation in Flavor and Fragrance Industry
Department of Biology, School of Science, Al-zahra University, Vanak, Tehran, Iran
Jamshid Fooladi
Department of Biology, School of Science, Al-zahra University, Vanak, Tehran, Iran
Mansour Bayat
Department of Science and Research, Islamic Azad University, Tehran, Iran