Research Article
A Novel Technique for Wastewater Treatment by Contact Glow-discharge Electrolysis
Collage of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, Peoples Republic of China
In the late of 20th century, plasma chemistry as a new branch of subject has emerged and attracted many researchers moving their focus on this field (Zhao, 1993; Chen, 2001). The basic principle is, roughly, taking the energetic particles in plasma to drive the chemical reaction. According to the difference between the electronic temperature (Te) and ionic temperature (Ti) the plasma can be divided into two kinds: one is called the thermal plasma or equilibrium plasma in which all of particles have the same temperature, i.e., Te = Ti ; the other is said to be non-equilibrium plasma or nonthermal plasma (or cold plasma), commonly, called low-temperature plasma, in which Te >> Ti. Based on the discharge generation, the low-temperature plasma can be also classified into many types such as glow discharge, corona discharge, dielectric barrier discharge, radio frequency discharge, microwave discharge and so on. All of these discharges are obtained under the gaseous phase with the special conditions. Wei and Liu (1995) and Lin (1999) reported the characteristic and parameters of DC glow discharge plasma from the point of view of physics and mathematics. The electrons in cathode sheath can be accelerated to get energy or to lose energy by non-elastic collision. Based on the Monte Carlo Simulation, the electron mean energy (ε) in spatial distribution may divide into three sections, i.e, low-energy electrons (ε < 25 eV), middle-energy electrons (25 eV < ε < 100 eV) and high-energy electrons (ε > 100 eV). The behavior of energetic electrons is very important to plasma chemistry, because these energetic electrons could collide further the other molecules to create a lot of new reactions. An overview for atmospheric pressure discharges producing nonthermal plasma has been made by Napartovich (2001).
Most of the chemical reactions are carried out in aqueous solution. To study aqueous solution chemistry by means of low-temperature plasma is more lure rather than in gaseous phase. One of the applications in this field is to remove the organic substances from water or to treat wastewater. Some review papers have summarized the progresses (Malik et al., 2001; Gao et al., 2001a; Wu, 1999; Bian et al., 2003). It is worth notice that Pacific Northwest National Laboratory for the US Department of Energy is an important laboratory in this field and often reports the new results (http://www.pnl.gov/aoam/researchs/).
In marked contrast to the extensive studies of corona discharge and dielectric barrier discharge, very few papers on the contact glow-discharge electrolysis have been reported. Thus, this mini-review will be just limited in the contact glow discharge electrolysis and its application to degradation of aqueous organic compounds. Of course, some basic principle will be cited from the others.
NON-FARADAIC ELECTROLYSIS
The most well known theory in electrochemistry is Faraday’s law of electrolysis. It describes accurately the relations between the amount of electricity passed through a cell and the chemical reactions that occur during the electrolysis. However, Hickling and Ingram (Hickling and Ingram, 1964a,b; Denaro and Hickling, 1958; Hickling and Linacre,1954) found that in the contact glow-discharge electrolysis a remarkable deviation of the chemical yield from that expected on the basis of Faraday’s law was obtained. That is, the contact glow-discharge electrolysis is not obeyed the Faraday’s law. The chemical yield, sometime, is more than that expected on the basis of Faraday’s law, implying some new reactions could be created in the glow-discharge electrolysis.
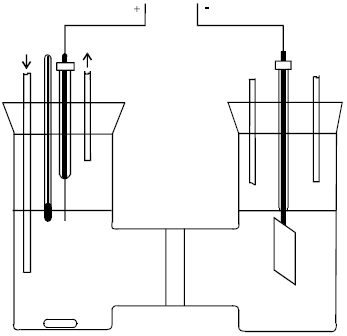 | |
| Fig. 1: | Contact glow-discharge electrolytic cell |
Sengupta et al. (1995, 1997, 1998a,b), Sengupta and Singh, (1994) also studied in detail the non- faradaic behavior of anodic contact glow-discharge electrolysis. For more understanding what the contact glow-discharge electrolysis is, a simplest electrolytic cell is shown in Fig. 1.
Where the left half-cell is anodic zone, a needle platinum-wire sealed in a Pyrex tube as an anode hanging above the electrolytic solution just contacting the surface of solution. The right half-cell is cathodic zone, a platinum-net (or stainless steel, or nickel foil, or graphitic piece) immersing into the solution as a cathode. Because this is an electrolysis process, the electrolytes must be used to conduct the current. For the convenience of study in various conditions, the inert-electrolytes such as KSO4 or NaSO4 are often accepted. As we know that in normal electrolysis both anode and cathode are approximately same in size and placed in parallel direction to keep the electric field uniformity. However, if a needle platinum-wire anode of much smaller surface area than the cathode was used, the uniform electric field would be destroyed to cause non-faradaic electrolysis, even glow-discharge appeared when the applied voltage was enough high. A typical current-voltage graph (Gao et al., 2004a) was given in Fig. 2 and it falls several sections. There is a linear section below 200 V (A→B), where the conventional electrolysis occurred with small bubbles of gas leaving the anode. Between 200 and 380 V (B→C), the readings of both current and voltage fluctuated widely so that the readings are less important for the drawing in Fig. 2.
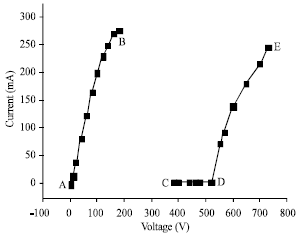 | |
| Fig. 2: | Characteristic current-voltage graph |
 | |
| Fig. 3a: | Photograph of glow discharge on the surface of aqueous solution |
 | |
| Fig. 3b: | Photograph of glow discharge in aqueous solution |
From 380 to 520 V (C→D), the current and voltage readings become suddenly stabilized and a smooth pale sheath of vapor appeared and flashes of light were bigger. Over the voltage of 520 V (D→E), a true glow discharge will be started and the intensity increases with rising voltage. This section should be a real plasma region. The above four regions have been proved by Hickling and Ingram (1994b) even though the data in curve in each paper are not the same completely. However, the change trends are the same completely.
Now, we have a more clear understanding on the term of contact glow-discharge electrolysis plasma. The word contact means that in the electrolysis process the needle platinum-wire anode contacts just the surface of solution. The word electrolysis implies that both Faraday and Non-faradaic electrolysis exist in this process. Due to this is an electrolysis, a little of inorganic ions (e.g., K+, Na+, SO42¯ ions etc.) are required.
Roughly speaking, glow discharge plasma was taken place in gas phase (inert gas) under high voltage. The plasma induced by the contact glow-discharge electrolysis is of more benefit to solution chemistry rather than by the other ways. This kind of plasma was occurred above the surface of solution at first and then moved into the solution. For improving the efficiency, Gao et al. (2004b) suggested that both anode and cathode are submersed completely in the solution to start discharge. The plasma induced by the two ways is photographed in Fig. 3A and B. In the contact way the glow discharge occurs in the interface between gaseous phase and solution. And then the energetic particles in plasma transfer into aqueous solution to collide the other molecules. Whereas, due to the energetic particles produced in the submersed way can directly react with the circumjacent molecules, the efficiency for solution reaction would be better than the former.
EXPERIMENTAL APPARATUS
Various setups have been designed for the different purpose in the study. A H-type divided cell, which was divided into two compartments by a sintered glass disk as a bridge showing in Fig. 1, was often easy used to examine the non-faradaic behavior of contact glow-discharge electrolysis Sengupta et al., 1998b). Other necessary facilities for collecting products can be connected with the compartments. In the case of studying electrolytic behavior, a suitable buffer solution and some inorganic salts would be required to keep the pH value or ionic strength constant. In addition, the power supply, stir and controlling-temperature unit are also need. In the degradation of organic substances in aqueous, the commonly used setups (Gao and Shen, 2005) whether contact or submersed way, are showing in Fig. 4 and 5. For the special purpose, various setups were often used. For example, a multi-electrode setup has been reported for degradation of Alizarin Red (Gao et al., 2005).
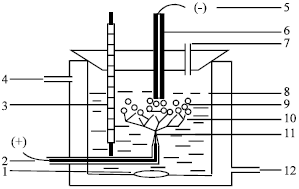 | |
| Fig. 4: | The sketch of contact glow discharge electrolysis, 1. Stir; 2. Anode; 3. Thermometer; 4. Cooling water outlet; 5. Cathode; 6. Graphite; 7. Sampling port; 8. Solution level; 9. Gas bubbles; 10. Glow discharge area; 11. Platinum wire; 12. Cooling water inlet |
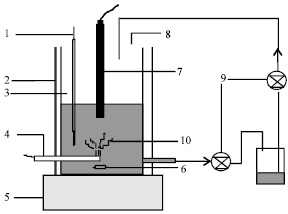 | |
| Fig. 5: | The sketch of submersed glow discharge electrolysis, 1. Thermometer; 2. Cooling jacket; 3. Reactor; 4. Anode; 5. Stirring machine; 6. Stir; 7. Cathode; 8. Hole; 9. Pump; 10. Glow discharge |
 | |
| Fig. 6: | A photo of drinking water clearer. Upper part is a glow discharge setup; Lower part is a power supply |
However, their basic principles are not changed, still being glow-discharge electrolysis. In the really practical application, only one setup for cleaning the cellar water for family expenses has been reported, showing in Fig. 6 (Gao and Shen, 2005).
DEGRADATION OF ORGANIC SUBSTANCES IN WATER
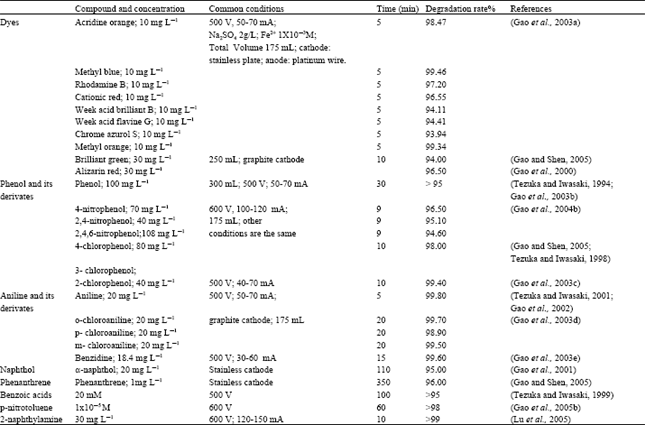
The removal of organic compounds in water is a hot topic. Many techniques such as advanced oxidation (Zhao et al., 2004) and low-temperature plasma have been investigated widely (Zhou et al., 2004; Zhu et al., 2002). In contrast to the extensive studies of water treatment techniques, relatively few papers of application of the glow-discharge electrolysis have been reported. As a new technique used in the purification of water, Tezuka and Iwasak (Tezuka and Iwasaki, 1997, 1998, 1999, 2001) reported the degradation mechanism of phenols, chlorophenols, benzoic acids and aniline in aqueous solution with contact glow-discharge electrolysis. Gai and Dong (2005) reported the degradation of auramine. Gao et al. (2001b, 2002, 2003a-e, 2005a,b), Lu et al. (2005a) studied systemically the degradation of dyes and some organic pollutants in aqueous solution by means of contact (or submersed) glow-discharge electrolysis. It was found that the final products were decomposed into carbon dioxide, water and corresponding inorganic ions. Generally, high degradation rate can be obtained in shorter period, just showing in Table 1.
The intensity of plasma induced by glow-discharge electrolysis was changed with changing voltage and/or current. In fact, the degradation rate of a substance in aqueous was affected by many factors such as electrode materials and shape, distance between both electrodes (even the direction of electrodes), concentration and property of electrolyte and/or contaminated substance, pH and temperature and so on. Fe2+ ion is a very important catalyst in the degradation just like Fenton reaction. The examination of degradation processes was made by means of HPLC, GC-MS, COD and UV-spectrometry. Generally speaking, there is a long way from an experimental study in laboratory to the application in industry for glow-discharge electrolysis technique except in the cellar water for family use (Gao and Shen, 2005). However, its potential advantages have been emerged in wastewater treatment such as high efficiency and simple equipment.
| Table 1: | Degradation of some organic compounds by glow discharge electrolysis |
 | |
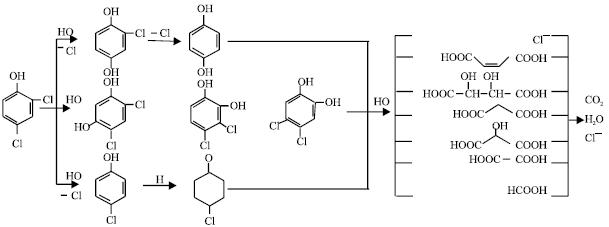 | |
| Fig. 7: | Proposed reaction pathway for 2, 4-DCP by glow- discharge electrolysis |
It is interest notice that this technique can also be used in hydrometallurgy. For example, Irving (2003) said that using this technology could generate activated water, which is a catalyst that can enhance many chemical reactions by a factor of 40-50 times. He also said: A cyanide leaching process might require 72-82 h for a 65 to 85% recovery of gold or silver. However, this same leaching process might be accomplished in three to four hours if activated water were substituted for river or rainwater. Further, recoveries have been found to be in the 95 to 99.5% range when the leaching was performed with activated water.
MECHANISM
While the contact glow-discharge electrolysis occurs, the Joule effect, highly energetic electron, activated species, free radical and UV-light are often accompanied to form plasma. Commonly, it should be called plasma induced by glow-discharge electrolysis, differing from the gaseous plasma. The electrons in discharge process are easy accelerated to gain enough energy and to collide further the other molecules. In aqueous the effective collision creates a lot of activated species such as OH*, H* and HO2* free radicals. Based on the analysis of intermediate products in degradation of organic compounds in aqueous solution, for aromatic compounds, the following steps occur in sequence, i.e., hydroxylation, cleaving ring, oxidation and formation of carboxylic acid with smaller molecular weight. And the final products will be CO2, H2O and inorganic ions. In which the OH* radical plays an important role. This is why in the degradation process the pH of solution observed often decreases. For example, a scheme degradation of 2,4-dichlorophenol (2,4-DCP) was shown in Fig. 7 (Lu et al., 2005a).
The theoretical studies of glow-discharge electrolysis continue to be an interesting field. However, the application studies are more attraction for searchers who are working in arid region, in which cellar water was used to be dinking water (An and Zhang, 2002; Yang et al., 2001; Ma et al., 2000). A small setup showing in Fig. 6 is a cellar-water purifier designed for family use. 5 L of water can be purified within 3 minutes to match the required standard of drinking water. It may be suitable for purification of decentralized water in future.
This study was supported in part by the Project of Key Science and Technology of Education Ministry (00250), the Natural Science Foundation of Gansu Province (3ZS041-A25-028) and the Invention Project of Science and Technology (KJCXGC-01, NWNU), China.