Research Article
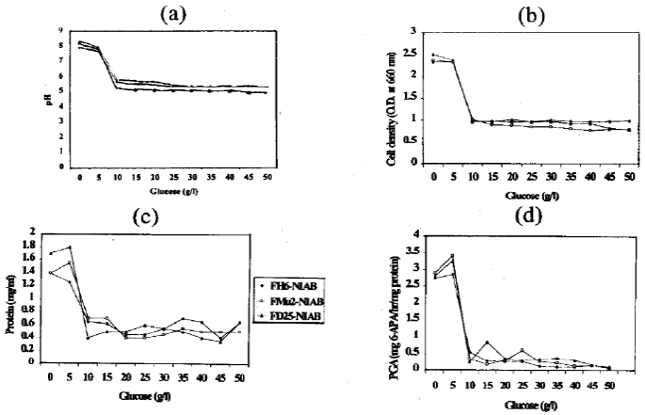
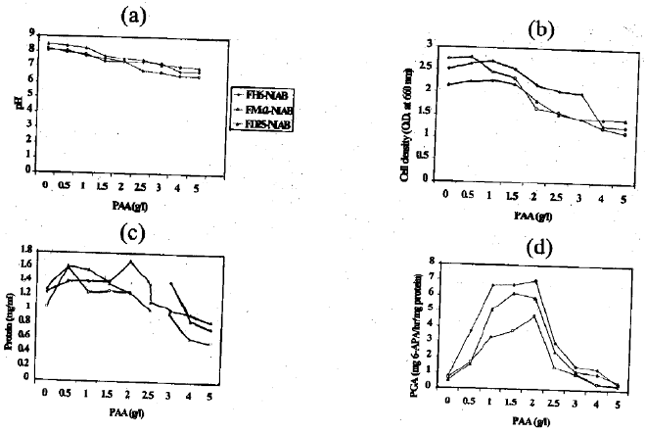
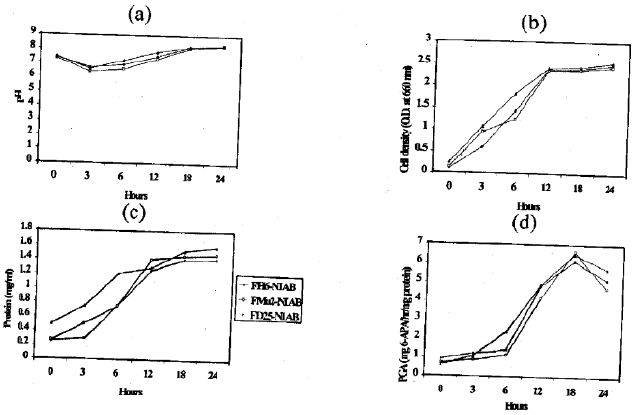
Kinetic Study of Growth and Penicillin G Acylase Production by Escherichia coli
Nuclear Institute for Agriculture and Biology (NIAB), P.O. Box 128, Jhang Road, Faisalabad, Pakistan
Muhammad Salih Ahmad
Nuclear Institute for Agriculture and Biology (NIAB), P.O. Box 128, Jhang Road, Fap Isalabad, Pakistan