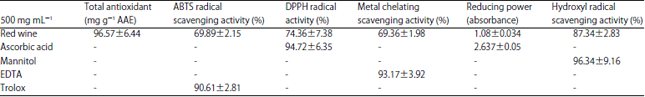
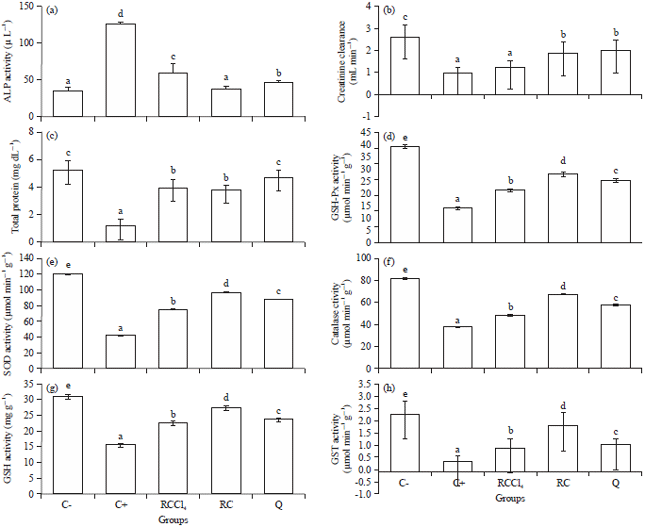
Research Article
Ameliorative Potential of Alcoholic Red Wine Against CCl4-induced Renalotoxicity in Albino Rats
Department of Biochemistry, Federal University of Technology Akure, Ondo State, Nigeria
G.O. Oladipo
Department of Biochemistry, Federal University of Technology Akure, Ondo State, Nigeria